Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events
- PMID: 26819745
- PMCID: PMC4728763
- DOI: 10.1186/s40780-015-0035-2
Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events
Abstract
Background: Bisphosphonates (BPs) are potent antiresorptive agents used to treat osteoporosis and the complications associated with malignant bone metastasis. The aim of this study was to evaluate the incidence of bisphosphonate-related osteonecrosis of the jaw (BRONJ) using the Japanese Adverse Drug Event Report (JADER) database. In particular, we focused on the time-to-onset profile of BRONJ.
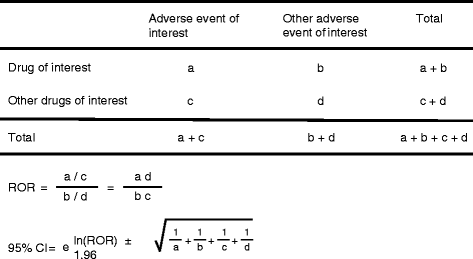
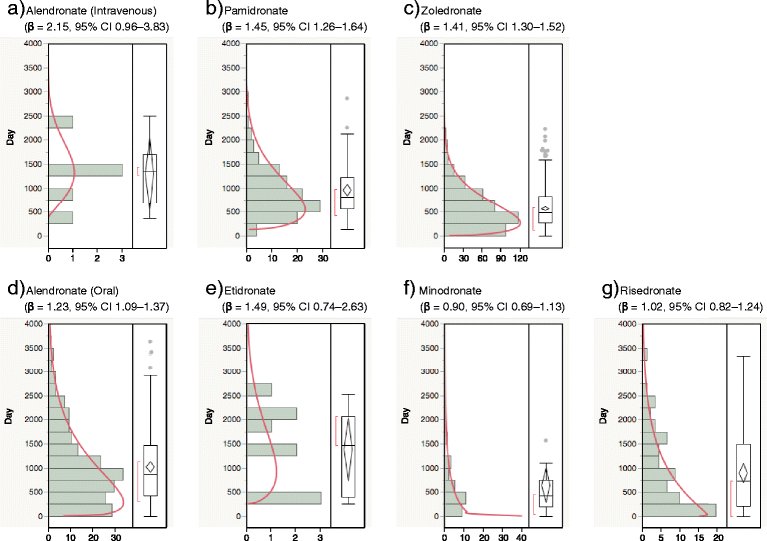
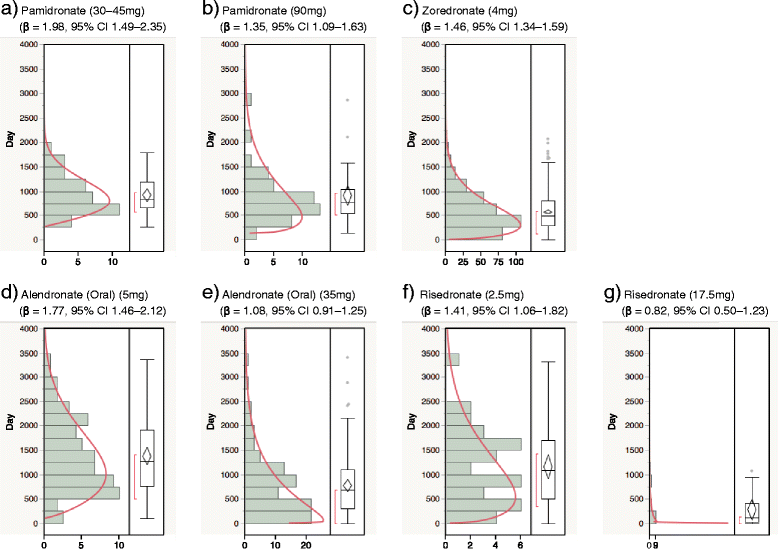
Findings: We analyzed reports of BRONJ in the JADER database and calculated the reporting odds ratio (ROR) of BPs potentially associated with BRONJ. We applied the weibull shape parameter to time-to-event data in JADER. The drugs selected for this investigation were seven BPs approved in Japan (alendronate [intraveneous, I.V.], pamidronate, and zoledronate as I.V. BPs; and alendronate (oral), etidronate, minodronate, and risedronate as oral BPs). We analyzed reports of BRONJ events associated with BPs in the JADER database from April 2004 to November 2014. The median value of BRONJ cases caused by alendronate (I.V.), pamidronate, zoledronate, alendronate (oral), etidronate, minodronate, and risedronate were 1342, 812, 486, 863, 1461, 432, and 730 days, respectively. The lower 95 % confidence interval of the Weibull-shape parameter β for I.V. BPs (pamidronate and zoledronate) exceeded 1. The risk of BRONJ with I.V. BPs increased over time.
Conclusion: Thus, the incidence of BRONJ with BP treatment should be closely monitored for a 3-year period. Further studies are required to draw conclusions, and we believe that this information about BRONJ induced by BPs will prove beneficial to patients and pharmacists.
Keywords: Bisphosphonate; Japanese adverse drug event report database; Osteonecrosis of jaw.
Figures
Similar articles
-
Zoledronate and osteonecrosis of the jaw in osteoporosis: incidence and risk factors. Analysis of the French Pharmacovigilance Database.Joint Bone Spine. 2023 Dec;90(6):105599. doi: 10.1016/j.jbspin.2023.105599. Epub 2023 Jun 2. Joint Bone Spine. 2023. PMID: 37271278
-
Evaluation of medication-related osteonecrosis of the jaw using the Japanese Adverse Drug Event Report database.Ther Clin Risk Manag. 2018 Dec 24;15:59-64. doi: 10.2147/TCRM.S176620. eCollection 2019. Ther Clin Risk Manag. 2018. PMID: 30636879 Free PMC article.
-
Real-world study of antiresorptive-related osteonecrosis of jaw based on the US food and drug administration adverse event reporting system database.Front Pharmacol. 2022 Oct 19;13:1017391. doi: 10.3389/fphar.2022.1017391. eCollection 2022. Front Pharmacol. 2022. PMID: 36339548 Free PMC article.
-
[Basic Studies on the Mechanism, Prevention, and Treatment of Osteonecrosis of the Jaw Induced by Bisphosphonates].Yakugaku Zasshi. 2020;140(1):63-79. doi: 10.1248/yakushi.19-00125. Yakugaku Zasshi. 2020. PMID: 31902887 Review. Japanese.
-
Underlying Mechanisms and Therapeutic Strategies for Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ).Biol Pharm Bull. 2017;40(6):739-750. doi: 10.1248/bpb.b16-01020. Biol Pharm Bull. 2017. PMID: 28566618 Review.
Cited by
-
Efficacy and safety of minodronate in the treatment of postmenopausal osteoporosis with low back pain: a single-centre, randomized and open-label controlled trial.Trials. 2024 Aug 13;25(1):534. doi: 10.1186/s13063-024-08364-7. Trials. 2024. PMID: 39135126 Free PMC article.
-
Analysis of the risk of oncological adverse events associated with infliximab in combination with azathioprine compared to monotherapy: insights from the FAERS database.Front Pharmacol. 2025 Jan 8;15:1507196. doi: 10.3389/fphar.2024.1507196. eCollection 2024. Front Pharmacol. 2025. PMID: 39845804 Free PMC article.
-
Interventions for managing medication-related osteonecrosis of the jaw.Cochrane Database Syst Rev. 2017 Oct 6;10(10):CD012432. doi: 10.1002/14651858.CD012432.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jul 12;7:CD012432. doi: 10.1002/14651858.CD012432.pub3. PMID: 28983908 Free PMC article. Updated.
-
Adverse Event Profiles of the Third-Generation Aromatase Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS.Biomedicines. 2024 Aug 1;12(8):1708. doi: 10.3390/biomedicines12081708. Biomedicines. 2024. PMID: 39200174 Free PMC article.
-
Antihypertensive drug-associated adverse events in osteoarthritis: a study of a large real-world sample based on the FAERS database.Front Pharmacol. 2024 Sep 2;15:1404427. doi: 10.3389/fphar.2024.1404427. eCollection 2024. Front Pharmacol. 2024. PMID: 39286630 Free PMC article.
References
-
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw −2014 update. J Oral Maxillofac Surg. 2014;72:1938–56. doi: 10.1016/j.joms.2014.04.031. - DOI - PubMed
-
- Mariotti A. Bisphosphonates and osteonecrosis of the jaws. J Dent Educ. 2008;72:919–29. - PubMed
-
- Umetsu R, Nishibata Y, Abe J, Suzuki Y, Hara H, Nagasawa H, et al. Evaluation of the association between the use of oral anti-hyperglycemic agents and hypoglycemia in Japan by data mining of the Japanese Adverse Drug Event Report (JADER) database. Yakugaku Zasshi. 2014;134:299–304. doi: 10.1248/yakushi.13-00225. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

