A multicentre, randomised, controlled, open-label pilot study on the feasibility of discontinuation of adalimumab in established patients with rheumatoid arthritis in stable clinical remission
- PMID: 26819752
- PMCID: PMC4716561
- DOI: 10.1136/rmdopen-2015-000133
A multicentre, randomised, controlled, open-label pilot study on the feasibility of discontinuation of adalimumab in established patients with rheumatoid arthritis in stable clinical remission
Abstract
Objectives: Treatment with tumour necrosis factor (TNF) blockers, once started as therapy for rheumatoid arthritis (RA), is usually continued indefinitely. The aim of this trial was to assess the possibility of discontinuing treatment with adalimumab (ADA) while maintaining remission in patients with RA with established disease in stable remission on combination therapy with ADA and methotrexate (MTX).
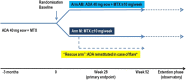
Methods: In a randomised, controlled, open-label pilot study of patients with RA in stable remission treated with ADA+MTX, patients were randomised in a 1:1 ratio to continue with ADA plus MTX (arm AM) or MTX monotherapy (arm M) for 52 weeks. Flare was defined as Disease Activity Score (DAS28) ≥2.6 or a change in DAS28 (ΔDAS28) of >1.2 from baseline at any time. Patients in arm M with a flare restarted ADA. The primary end point was the proportion of patients in remission at week 28.
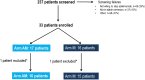
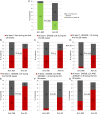
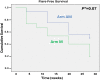
Results: 31 patients were enrolled in the study and randomised to arm AM (n=16) or arm M (n=15). At 28 weeks, 15/16 patients (94%) and 5/15 patients (33%) in arms AM and M, respectively, were in remission (p=0.001). During the first 28 weeks, 50% (8/16) in the AM arm and 80% (12/15) in the M arm had a flare (p=0.08). The number of patients in the AM and M arms with ≥1 ΔDAS28 >1.2 during the first 28 weeks was 1/16 (6%) and 8/15 (53%), respectively (p=0.005).
Conclusions: In this study, remission was rarely maintained in patients with long-standing disease who discontinued ADA. Discontinuation may be feasible in only a minority of patients with established RA in stable clinical remission.
Trial registration number: NCT00808509.
Keywords: Anti-TNF; Rheumatoid Arthritis; Treatment.
Figures
Similar articles
-
Adalimumab discontinuation in patients with early rheumatoid arthritis who were initially treated with methotrexate alone or in combination with adalimumab: 1 year outcomes of the HOPEFUL-2 study.RMD Open. 2016 Feb 18;2(1):e000189. doi: 10.1136/rmdopen-2015-000189. eCollection 2016. RMD Open. 2016. PMID: 26925252 Free PMC article.
-
Low disease activity for up to 3 years after adalimumab discontinuation in patients with early rheumatoid arthritis: 2-year results of the HOPEFUL-3 Study.Arthritis Res Ther. 2017 Mar 14;19(1):56. doi: 10.1186/s13075-017-1264-6. Arthritis Res Ther. 2017. PMID: 28288682 Free PMC article.
-
Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study.Ann Rheum Dis. 2015 Feb;74(2):389-95. doi: 10.1136/annrheumdis-2013-204016. Epub 2013 Nov 28. Ann Rheum Dis. 2015. PMID: 24288014 Free PMC article. Clinical Trial.
-
Is it possible to withdraw biologics from therapy in rheumatoid arthritis?Clin Ther. 2013 Dec;35(12):2028-35. doi: 10.1016/j.clinthera.2013.10.008. Epub 2013 Nov 28. Clin Ther. 2013. PMID: 24290736 Review.
-
[Adalimumab (Humira)--efficacy in rheumatoid arthritis treatment with particular reference to working ability].Reumatizam. 2008;55(2):62-7. Reumatizam. 2008. PMID: 19024278 Review. Croatian.
Cited by
-
Optimizing treatment with tumour necrosis factor inhibitors in rheumatoid arthritis-a proof of principle and exploratory trial: is dose tapering practical in good responders?Rheumatology (Oxford). 2017 Nov 1;56(11):2004-2014. doi: 10.1093/rheumatology/kex315. Rheumatology (Oxford). 2017. PMID: 28968858 Free PMC article. Clinical Trial.
-
Disease activity-guided dose optimization including discontinuation of TNF inhibitors in rheumatoid arthritis is effective for up to 10 years: an observational follow-up of the DRESS study.Rheumatology (Oxford). 2025 Feb 1;64(2):533-540. doi: 10.1093/rheumatology/keae103. Rheumatology (Oxford). 2025. PMID: 38346712 Free PMC article.
-
Dosing down with biologic therapies: a systematic review and clinicians' perspective.Rheumatology (Oxford). 2017 Nov 1;56(11):1847-1856. doi: 10.1093/rheumatology/kew464. Rheumatology (Oxford). 2017. PMID: 28339632 Free PMC article.
-
The first case of biological therapy discontinuation after a complete remission induced by maintenance therapy with adalimumab for refractory ulcerative colitis.J Clin Med Res. 2015 Feb;7(2):118-21. doi: 10.14740/jocmr1991w. Epub 2014 Nov 19. J Clin Med Res. 2015. PMID: 25436030 Free PMC article.
-
Switching from TNFα inhibitor to tacrolimus as maintenance therapy in rheumatoid arthritis after achieving low disease activity with TNFα inhibitors and methotrexate: 24-week result from a non-randomized, prospective, active-controlled trial.Arthritis Res Ther. 2021 Jul 8;23(1):182. doi: 10.1186/s13075-021-02566-z. Arthritis Res Ther. 2021. PMID: 34233727 Free PMC article. Clinical Trial.
References
-
- Breedveld FC, Weisman MH, Kavanaugh AF et al. . The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 - DOI - PubMed
-
- Lipsky PE, van der Heijde DM, St Clair EW et al. , Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343:1594–602. 10.1056/NEJM200011303432202 - DOI - PubMed
-
- Quinn MA, Conaghan PG, O'Connor PJ et al. . Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:27–35. 10.1002/art.20712 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
