EEG Radiotelemetry in Small Laboratory Rodents: A Powerful State-of-the Art Approach in Neuropsychiatric, Neurodegenerative, and Epilepsy Research
- PMID: 26819775
- PMCID: PMC4706962
- DOI: 10.1155/2016/8213878
EEG Radiotelemetry in Small Laboratory Rodents: A Powerful State-of-the Art Approach in Neuropsychiatric, Neurodegenerative, and Epilepsy Research
Abstract
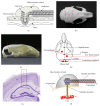
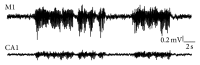
EEG radiotelemetry plays an important role in the neurological characterization of transgenic mouse models of neuropsychiatric and neurodegenerative diseases as well as epilepsies providing valuable insights into underlying pathophysiological mechanisms and thereby facilitating the development of new translational approaches. We elaborate on the major advantages of nonrestraining EEG radiotelemetry in contrast to restraining procedures such as tethered systems or jacket systems containing recorders. Whereas a main disadvantage of the latter is their unphysiological, restraining character, telemetric EEG recording overcomes these disadvantages. It allows precise and highly sensitive measurement under various physiological and pathophysiological conditions. Here we present a detailed description of a straightforward successful, quick, and efficient technique for intraperitoneal as well as subcutaneous pouch implantation of a standard radiofrequency transmitter in mice and rats. We further present computerized 3D-stereotaxic placement of both epidural and deep intracerebral electrodes. Preoperative preparation of mice and rats, suitable anaesthesia, and postoperative treatment and pain management are described in detail. A special focus is on fields of application, technical and experimental pitfalls, and technical connections of commercially available radiotelemetry systems with other electrophysiological setups.
Figures






Comment in
-
Sampling rate, signal bandwidth and related pitfalls in EEG analysis.J Neurosci Methods. 2016 Aug 1;268:53-5. doi: 10.1016/j.jneumeth.2016.05.010. Epub 2016 May 9. J Neurosci Methods. 2016. PMID: 27172844
Similar articles
-
Non-restraining EEG Radiotelemetry: Epidural and Deep Intracerebral Stereotaxic EEG Electrode Placement.J Vis Exp. 2016 Jun 25;(112):54216. doi: 10.3791/54216. J Vis Exp. 2016. PMID: 27404845 Free PMC article.
-
Electrocorticographic and deep intracerebral EEG recording in mice using a telemetry system.Brain Res Brain Res Protoc. 2005 Apr;14(3):154-64. doi: 10.1016/j.brainresprot.2004.12.006. Brain Res Brain Res Protoc. 2005. PMID: 15795169
-
Long-term Continuous EEG Monitoring in Small Rodent Models of Human Disease Using the Epoch Wireless Transmitter System.J Vis Exp. 2015 Jul 21;(101):e52554. doi: 10.3791/52554. J Vis Exp. 2015. PMID: 26274779 Free PMC article.
-
Evaluation and applications of radiotelemetry in small laboratory animals.Physiol Genomics. 2003 May 13;13(3):197-205. doi: 10.1152/physiolgenomics.00164.2002. Epub 2003 May 13. Physiol Genomics. 2003. PMID: 12746464 Review.
-
"All that spikes is not fits", mistaking the woods for the trees: the interictal spikes--an "EEG chameleon" in the interface disorders of brain and mind: a critical review.Clin EEG Neurosci. 2009 Oct;40(4):245-61. doi: 10.1177/155005940904000407. Clin EEG Neurosci. 2009. PMID: 19780346 Review.
Cited by
-
OSERR: an open-source standalone electrophysiology recording system for rodents.Sci Rep. 2020 Oct 12;10(1):16996. doi: 10.1038/s41598-020-73797-4. Sci Rep. 2020. PMID: 33046761 Free PMC article.
-
Mouse Exploratory Behaviour in the Open Field with and without NAT-1 EEG Device: Effects of MK801 and Scopolamine.Biomolecules. 2024 Aug 15;14(8):1008. doi: 10.3390/biom14081008. Biomolecules. 2024. PMID: 39199395 Free PMC article.
-
Telemetric electroencephalography recording in anesthetized mice-A novel system using minimally-invasive needle electrodes with a wireless OpenBCI™ Cyton Biosensing Board.MethodsX. 2023 Apr 15;10:102187. doi: 10.1016/j.mex.2023.102187. eCollection 2023. MethodsX. 2023. PMID: 37424756 Free PMC article.
-
Enhanced hippocampal type II theta activity AND altered theta architecture in mice lacking the Cav3.2 T-type voltage-gated calcium channel.Sci Rep. 2021 Jan 13;11(1):1099. doi: 10.1038/s41598-020-79763-4. Sci Rep. 2021. PMID: 33441788 Free PMC article.
-
Emerging Electroencephalographic Biomarkers to Improve Preclinical to Clinical Translation in Alzheimer's Disease.Front Aging Neurosci. 2022 Feb 17;14:805063. doi: 10.3389/fnagi.2022.805063. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250541 Free PMC article. Review.
References
-
- Kramer K., Kinter L., Brockway B. P., Voss H.-P., Remie R., Van Zutphen B. L. M. The use of radiotelemetry in small laboratory animals: recent advances. Contemporary Topics in Laboratory Animal Science. 2001;40(1):8–16. - PubMed
-
- Kramer K., Grimbergen J. A., van der Gracht L., van Iperen D. J., Jonker R. J., Bast A. The use of telemetry to record electrocardiogram and heart rate in freely swimming rats. Methods and Findings in Experimental and Clinical Pharmacology. 1995;17(2):107–112. - PubMed
-
- Kramer K., Remie R. Measuring blood pressure in small laboratory animals. Methods in Molecular Medicine. 2005;108:51–62. - PubMed
-
- Kramer K., van Acker S. A. B. E., Voss H.-P., Grimbergen J. A., van der Vijgh W. J. F., Bast A. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. Journal of Pharmacological and Toxicological Methods. 1993;30(4):209–215. doi: 10.1016/1056-8719(93)90019-B. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

