Enhancement of the Oral Bioavailability of Fexofenadine Hydrochloride via Cremophor(®) El-Based Liquisolid Tablets
- PMID: 26819931
- PMCID: PMC4729349
- DOI: 10.15171/apb.2015.077
Enhancement of the Oral Bioavailability of Fexofenadine Hydrochloride via Cremophor(®) El-Based Liquisolid Tablets
Abstract
Purpose: The current work aimed to develop promising Fexofenadine hydrochloride (FXD) liquisolid tablets able to increase its oral bioavailability and shorten time to reach maximum plasma concentrations (Tmax).
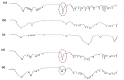
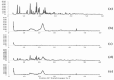
Methods: Eighteen liquisolid powders were developed based on 3 variables; (i) vehicle type [Propylene glycol (PG) or Cremophor(®) EL (CR)], (ii) carrier [Avicel(®) PH102] to coat [Aerosil(®) 200] ratio (15, 20, 25) and (iii) FXD concentration in vehicle (30, 35, 40 %, w/w). Pre-compression studies involved identification of physicochemical interactions and FXD crystallinity (FT-IR, DSC, XRD), topographic visualization (SEM) and estimation of flow properties (angle of repose, Carr's index, Hausner's ratio). CR-based liquisolid powders were compressed as liquisolid tablets (LST 9 - 18) and evaluated for weight-variation, drug-content, friability-percentage, disintegration-time and drug-release. The pharmacokinetics of LST-18 was evaluated in healthy volunteers relative to Allegra(®) tablets.
Results: Pre-compression studies confirmed FXD dispersion in vehicles, conversion to amorphous form and formation of liquisolid powders. CR-based liquisolid powders showed acceptable-to-good flow properties suitable for compaction. CR-based LSTs had appropriate physicochemical properties and short disintegration times. Release profile of LST-18 showed a complete drug release within 5 min.
Conclusion: LST-18 succeeded in increasing oral FXD bioavailability by 62% and reducing Tmax to 2.16 h.
Keywords: Cremophor® EL; Fexofenadine hydrochloride; Liquisolid tablets; Oral bioavailability; Pharmacokinetics.
Figures
Similar articles
-
Phenylalanine-free taste-masked orodispersible tablets of fexofenadine hydrochloride: development, in vitro evaluation and in vivo estimation of the drug pharmacokinetics in healthy human volunteers.Pharm Dev Technol. 2015;20(5):528-39. doi: 10.3109/10837450.2014.882942. Epub 2014 Feb 3. Pharm Dev Technol. 2015. PMID: 24490806
-
Effects of liquisolid formulations on dissolution of naproxen.Eur J Pharm Biopharm. 2009 Nov;73(3):373-84. doi: 10.1016/j.ejpb.2009.08.002. Epub 2009 Aug 11. Eur J Pharm Biopharm. 2009. PMID: 19679184
-
Liquisolid technique to enhance and to sustain griseofulvin dissolution: effect of choice of non-volatile liquid vehicles.Int J Pharm. 2012 Sep 15;434(1-2):122-32. doi: 10.1016/j.ijpharm.2012.05.072. Epub 2012 Jun 5. Int J Pharm. 2012. PMID: 22677418
-
[Enhancing of drug bioavailability using liquisolid system formulation].Ceska Slov Farm. 2015 Jun;64(3):55-66. Ceska Slov Farm. 2015. PMID: 26400228 Review. Czech.
-
Liquisolid technology for dissolution rate enhancement or sustained release.Expert Opin Drug Deliv. 2010 Oct;7(10):1227-34. doi: 10.1517/17425247.2010.511173. Expert Opin Drug Deliv. 2010. PMID: 20731614 Review.
Cited by
-
Olanzapine Liquisolid Tablets Using Kolliphor EL with Improved Flowability and Bioavailability: In vitro and In vivo Characterization.Turk J Pharm Sci. 2024 Mar 25;21(1):52-61. doi: 10.4274/tjps.galenos.2023.05752. Turk J Pharm Sci. 2024. PMID: 38529549 Free PMC article.
-
Pharmaceutical Dispersion Techniques for Dissolution and Bioavailability Enhancement of Poorly Water-Soluble Drugs.Pharmaceutics. 2018 Jun 23;10(3):74. doi: 10.3390/pharmaceutics10030074. Pharmaceutics. 2018. PMID: 29937483 Free PMC article. Review.
-
Polymeric Mixed Micelle-Loaded Hydrogel for the Ocular Delivery of Fexofenadine for Treating Allergic Conjunctivitis.Polymers (Basel). 2024 Aug 7;16(16):2240. doi: 10.3390/polym16162240. Polymers (Basel). 2024. PMID: 39204460 Free PMC article.
-
Development of fexofenadine self-microemulsifying delivery systems: an efficient way to improve intestinal permeability.Ther Deliv. 2024;15(8):593-604. doi: 10.1080/20415990.2024.2363635. Epub 2024 Jun 28. Ther Deliv. 2024. PMID: 38941109 Free PMC article.
-
Dramatic improvement in dissolution rate of albendazole by a simple, one-step, industrially scalable technique.Res Pharm Sci. 2016 Dec;11(6):435-444. doi: 10.4103/1735-5362.194868. Res Pharm Sci. 2016. PMID: 28003836 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources