Enhancement of the Oral Bioavailability of Fexofenadine Hydrochloride via Cremophor(®) El-Based Liquisolid Tablets
- PMID: 26819931
- PMCID: PMC4729349
- DOI: 10.15171/apb.2015.077
Enhancement of the Oral Bioavailability of Fexofenadine Hydrochloride via Cremophor(®) El-Based Liquisolid Tablets
Abstract
Purpose: The current work aimed to develop promising Fexofenadine hydrochloride (FXD) liquisolid tablets able to increase its oral bioavailability and shorten time to reach maximum plasma concentrations (Tmax).
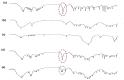
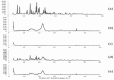
Methods: Eighteen liquisolid powders were developed based on 3 variables; (i) vehicle type [Propylene glycol (PG) or Cremophor(®) EL (CR)], (ii) carrier [Avicel(®) PH102] to coat [Aerosil(®) 200] ratio (15, 20, 25) and (iii) FXD concentration in vehicle (30, 35, 40 %, w/w). Pre-compression studies involved identification of physicochemical interactions and FXD crystallinity (FT-IR, DSC, XRD), topographic visualization (SEM) and estimation of flow properties (angle of repose, Carr's index, Hausner's ratio). CR-based liquisolid powders were compressed as liquisolid tablets (LST 9 - 18) and evaluated for weight-variation, drug-content, friability-percentage, disintegration-time and drug-release. The pharmacokinetics of LST-18 was evaluated in healthy volunteers relative to Allegra(®) tablets.
Results: Pre-compression studies confirmed FXD dispersion in vehicles, conversion to amorphous form and formation of liquisolid powders. CR-based liquisolid powders showed acceptable-to-good flow properties suitable for compaction. CR-based LSTs had appropriate physicochemical properties and short disintegration times. Release profile of LST-18 showed a complete drug release within 5 min.
Conclusion: LST-18 succeeded in increasing oral FXD bioavailability by 62% and reducing Tmax to 2.16 h.
Keywords: Cremophor® EL; Fexofenadine hydrochloride; Liquisolid tablets; Oral bioavailability; Pharmacokinetics.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources