Targeted Mybpc3 Knock-Out Mice with Cardiac Hypertrophy Exhibit Structural Mitral Valve Abnormalities
- PMID: 26819945
- PMCID: PMC4725593
- DOI: 10.3390/jcdd2020048
Targeted Mybpc3 Knock-Out Mice with Cardiac Hypertrophy Exhibit Structural Mitral Valve Abnormalities
Abstract
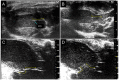
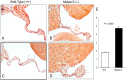
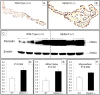
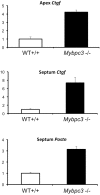
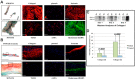
MYBPC3 mutations cause hypertrophic cardiomyopathy, which is frequently associated with mitral valve (MV) pathology. We reasoned that increased MV size is caused by localized growth factors with paracrine effects. We used high-resolution echocardiography to compare Mybpc3-null, heterozygous, and wild-type mice (n = 84, aged 3-6 months) and micro-CT for MV volume (n = 6, age 6 months). Mybpc3-null mice showed left ventricular hypertrophy, dilation, and systolic dysfunction compared to heterozygous and wild-type mice, but no systolic anterior motion of the MV or left ventricular outflow obstruction. Compared to wild-type mice, echocardiographic anterior leaflet length (adjusted for left ventricular size) was greatest in Mybpc3-null mice (1.92 ± 0.08 vs. 1.72 ± 0.08 mm, p < 0.001), as was combined leaflet thickness (0.23 ± 0.04 vs. 0.15 ± 0.02 mm, p < 0.001). Micro-CT analyses of Mybpc3-null mice demonstrated increased MV volume (0.47 ± 0.06 vs. 0.15 ± 0.06 mm3, p = 0.018) and thickness (0.35 ± 0.04 vs. 0.12 ± 0.04 mm, p = 0.002), coincident with increased markers of TGFβ activity compared to heterozygous and wild-type littermates. Similarly, excised MV from a patient with MYBPC3 mutation showed increased TGFβ activity. We conclude that MYBPC3 deficiency causes hypertrophic cardiomyopathy with increased MV leaflet length and thickness despite the absence of left ventricular outflow-tract obstruction, in parallel with increased TGFβ activity. MV changes in hypertrophic cardiomyopathy may be due to paracrine effects, which represent targets for therapeutic studies.
Keywords: TGFβ; hypertrophic cardiomyopathy; mitral valve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy Patients Without Severe Septal Hypertrophy: Implications of Mitral Valve and Papillary Muscle Abnormalities Assessed Using Cardiac Magnetic Resonance and Echocardiography.Circ Cardiovasc Imaging. 2015 Jul;8(7):e003132. doi: 10.1161/CIRCIMAGING.115.003132. Circ Cardiovasc Imaging. 2015. PMID: 26082555
-
Mitral stenosis and hypertrophic obstructive cardiomyopathy: An unusual combination.J Thorac Cardiovasc Surg. 2016 Apr;151(4):1044-8. doi: 10.1016/j.jtcvs.2015.11.048. Epub 2015 Dec 8. J Thorac Cardiovasc Surg. 2016. PMID: 26724969
-
Morphological determinants of echocardiographic patterns of mitral valve systolic anterior motion in obstructive hypertrophic cardiomyopathy.Circulation. 1993 May;87(5):1570-9. doi: 10.1161/01.cir.87.5.1570. Circulation. 1993. PMID: 8491013
-
Hypertrophic cardiomyopathy.Cardiol Clin. 1988 May;6(2):233-88. Cardiol Clin. 1988. PMID: 3066484 Review.
-
The mechanisms, diagnosis and management of mitral regurgitation in mitral valve prolapse and hypertrophic cardiomyopathy.Discoveries (Craiova). 2016 Jun 30;4(2):e61. doi: 10.15190/d.2016.8. Discoveries (Craiova). 2016. PMID: 32309580 Free PMC article. Review.
Cited by
-
The embryological basis of subclinical hypertrophic cardiomyopathy.Sci Rep. 2016 Jun 21;6:27714. doi: 10.1038/srep27714. Sci Rep. 2016. PMID: 27323879 Free PMC article.
-
Downregulation of GSTK1 Is a Common Mechanism Underlying Hypertrophic Cardiomyopathy.Front Pharmacol. 2016 Jun 14;7:162. doi: 10.3389/fphar.2016.00162. eCollection 2016. Front Pharmacol. 2016. PMID: 27378925 Free PMC article.
-
Biomechanical Impact of Pathogenic MYBPC3 Truncation Variant Revealed by Dynamically Tuning In Vitro Afterload.J Cardiovasc Transl Res. 2023 Aug;16(4):828-841. doi: 10.1007/s12265-022-10348-4. Epub 2023 Mar 6. J Cardiovasc Transl Res. 2023. PMID: 36877449 Free PMC article.
-
Does the Flow Know? Mitral Regurgitant Jet Direction and Need for Valve Repair in Hypertrophic Obstructive Cardiomyopathy.J Am Soc Echocardiogr. 2019 Mar;32(3):341-343. doi: 10.1016/j.echo.2019.01.005. J Am Soc Echocardiogr. 2019. PMID: 30827370 Free PMC article. No abstract available.
-
MYBPC3 Haplotype Linked to Hypertrophic Cardiomyopathy in Rhesus Macaques (Macaca mulatta).Comp Med. 2020 Oct 1;70(5):358-367. doi: 10.30802/AALAS-CM-19-000108. Epub 2020 Aug 4. Comp Med. 2020. PMID: 32753092 Free PMC article.
References
-
- Hagege A.A., Dubourg O., Desnos M., Mirochnik R., Isnard G., Bonne G., Carrier L., Guicheney P., Bouhour J.B., Schwartz K., Komajda M. Familial hypertrophic cardiomyopathy. Cardiac ultrasonic abnormalities in genetically affected subjects without echocardiographic evidence of left ventricular hypertrophy. Eur. Heart J. 1998;19:490–499. doi: 10.1053/euhj.1997.0735. - DOI - PubMed
-
- Maron M.S., Olivotto I., Harrigan C., Appelbaum E., Gibson C.M., Lesser J.R., Haas T.S., Udelson J.E., Manning W.J., Maron B.J. Mitral Valve Abnormalities Identified by Cardiovascular Magnetic Resonance Represent a Primary Phenotypic Expression of Hypertrophic Cardiomyopathy. Circulation. 2011;124:40–47. doi: 10.1161/CIRCULATIONAHA.110.985812. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
