Redox homeostasis: The Golden Mean of healthy living
- PMID: 26820564
- PMCID: PMC4732014
- DOI: 10.1016/j.redox.2016.01.010
Redox homeostasis: The Golden Mean of healthy living
Abstract
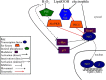
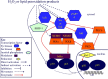
The notion that electrophiles serve as messengers in cell signaling is now widely accepted. Nonetheless, major issues restrain acceptance of redox homeostasis and redox signaling as components of maintenance of a normal physiological steady state. The first is that redox signaling requires sudden switching on of oxidant production and bypassing of antioxidant mechanisms rather than a continuous process that, like other signaling mechanisms, can be smoothly turned up or down. The second is the misperception that reactions in redox signaling involve "reactive oxygen species" rather than reaction of specific electrophiles with specific protein thiolates. The third is that hormesis provides protection against oxidants by increasing cellular defense or repair mechanisms rather than by specifically addressing the offset of redox homeostasis. Instead, we propose that both oxidant and antioxidant signaling are main features of redox homeostasis. As the redox shift is rapidly reversed by feedback reactions, homeostasis is maintained by continuous signaling for production and elimination of electrophiles and nucleophiles. Redox homeostasis, which is the maintenance of nucleophilic tone, accounts for a healthy physiological steady state. Electrophiles and nucleophiles are not intrinsically harmful or protective, and redox homeostasis is an essential feature of both the response to challenges and subsequent feedback. While the balance between oxidants and nucleophiles is preserved in redox homeostasis, oxidative stress provokes the establishment of a new radically altered redox steady state. The popular belief that scavenging free radicals by antioxidants has a beneficial effect is wishful thinking. We propose, instead, that continuous feedback preserves nucleophilic tone and that this is supported by redox active nutritional phytochemicals. These nonessential compounds, by activating Nrf2, mimic the effect of endogenously produced electrophiles (parahormesis). In summary, while hormesis, although globally protective, results in setting up of a new phenotype, parahormesis contributes to health by favoring maintenance of homeostasis.
Keywords: Hormesis; Kinetics; Oxidative stress; Phytochemicals; Signaling; Thiols.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Selye H. The Stress of Life. McGraw-Hill; New York: 1956.
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. - PubMed
-
- Cadenas E., Sies H. Oxidative stress: excited oxygen species and enzyme activity. Adv. Enzym. Regul. 1985;23:217–237. - PubMed
-
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

