Application of a Multiplex Quantitative PCR to Assess Prevalence and Intensity Of Intestinal Parasite Infections in a Controlled Clinical Trial
- PMID: 26820626
- PMCID: PMC4731196
- DOI: 10.1371/journal.pntd.0004380
Application of a Multiplex Quantitative PCR to Assess Prevalence and Intensity Of Intestinal Parasite Infections in a Controlled Clinical Trial
Abstract
Background: Accurate quantitative assessment of infection with soil transmitted helminths and protozoa is key to the interpretation of epidemiologic studies of these parasites, as well as for monitoring large scale treatment efficacy and effectiveness studies. As morbidity and transmission of helminth infections are directly related to both the prevalence and intensity of infection, there is particular need for improved techniques for assessment of infection intensity for both purposes. The current study aimed to evaluate two multiplex PCR assays to determine prevalence and intensity of intestinal parasite infections, and compare them to standard microscopy.
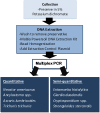
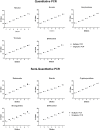
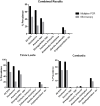
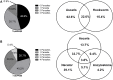
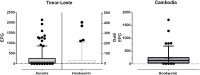
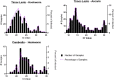
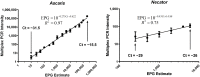
Methodology/principal findings: Faecal samples were collected from a total of 680 people, originating from rural communities in Timor-Leste (467 samples) and Cambodia (213 samples). DNA was extracted from stool samples and subject to two multiplex real-time PCR reactions the first targeting: Necator americanus, Ancylostoma spp., Ascaris spp., and Trichuris trichiura; and the second Entamoeba histolytica, Cryptosporidium spp., Giardia. duodenalis, and Strongyloides stercoralis. Samples were also subject to sodium nitrate flotation for identification and quantification of STH eggs, and zinc sulphate centrifugal flotation for detection of protozoan parasites. Higher parasite prevalence was detected by multiplex PCR (hookworms 2.9 times higher, Ascaris 1.2, Giardia 1.6, along with superior polyparasitism detection with this effect magnified as the number of parasites present increased (one: 40.2% vs. 38.1%, two: 30.9% vs. 12.9%, three: 7.6% vs. 0.4%, four: 0.4% vs. 0%). Although, all STH positive samples were low intensity infections by microscopy as defined by WHO guidelines the DNA-load detected by multiplex PCR suggested higher intensity infections.
Conclusions/significance: Multiplex PCR, in addition to superior sensitivity, enabled more accurate determination of infection intensity for Ascaris, hookworms and Giardia compared to microscopy, especially in samples exhibiting polyparasitism. The superior performance of multiplex PCR to detect polyparasitism and more accurately determine infection intensity suggests that it is a more appropriate technique for use in epidemiologic studies and for monitoring large-scale intervention trials.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Detection of selected intestinal helminths and protozoa at Hospital Universiti Sains Malaysia using multiplex real-time PCR.Trop Biomed. 2012 Sep;29(3):434-42. Trop Biomed. 2012. PMID: 23018507
-
Identification of human intestinal parasites affecting an asymptomatic peri-urban Argentinian population using multi-parallel quantitative real-time polymerase chain reaction.Parasit Vectors. 2015 Jul 17;8:380. doi: 10.1186/s13071-015-0994-z. Parasit Vectors. 2015. PMID: 26183074 Free PMC article.
-
The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village.Parasitol Int. 2014 Aug;63(4):597-603. doi: 10.1016/j.parint.2014.03.007. Epub 2014 Apr 4. Parasitol Int. 2014. PMID: 24704609
-
Evaluating measures to control intestinal parasitic infections.World Health Stat Q. 1992;45(2-3):168-79. World Health Stat Q. 1992. PMID: 1462652 Review.
-
Is real-time PCR-based diagnosis similar in performance to routine parasitological examination for the identification of Giardia intestinalis, Cryptosporidium parvum/Cryptosporidium hominis and Entamoeba histolytica from stool samples? Evaluation of a new commercial multiplex PCR assay and literature review.Clin Microbiol Infect. 2016 Feb;22(2):190.e1-190.e8. doi: 10.1016/j.cmi.2015.10.019. Epub 2015 Nov 6. Clin Microbiol Infect. 2016. PMID: 26548509 Review.
Cited by
-
Systematic Review of Strongyloides stercoralis Infection Diagnosis in Southeast Asia: Insights from Parasitological, Molecular, and Serological Approaches.Am J Trop Med Hyg. 2024 Aug 13;111(4):724-735. doi: 10.4269/ajtmh.23-0599. Print 2024 Oct 2. Am J Trop Med Hyg. 2024. PMID: 39137756
-
Evaluation of the Allplex™ GI-Helminth(I) Assay, the first marketed multiplex PCR for helminth diagnosis.Parasite. 2021;28:33. doi: 10.1051/parasite/2021034. Epub 2021 Apr 2. Parasite. 2021. PMID: 33812465 Free PMC article.
-
Molecular and Immnune Diagnosis: Further Testing for Human Strongyloidiasis.Mol Diagn Ther. 2018 Aug;22(4):485-491. doi: 10.1007/s40291-018-0340-1. Mol Diagn Ther. 2018. PMID: 29934882
-
Investigations into the association between soil-transmitted helminth infections, haemoglobin and child development indices in Manufahi District, Timor-Leste.Parasit Vectors. 2017 Apr 19;10(1):192. doi: 10.1186/s13071-017-2084-x. Parasit Vectors. 2017. PMID: 28424091 Free PMC article. Clinical Trial.
-
Diagnosis of protozoa diarrhoea in Campylobacter patients increases markedly with molecular techniques.PLOS Glob Public Health. 2023 May 30;3(5):e0001527. doi: 10.1371/journal.pgph.0001527. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37252910 Free PMC article.
References
-
- WHO (2013) Soil-transmitted helminth infection: Fact Sheet No. 366.
-
- Bethony J, Brooker S, Albonico M, Geiger S, Loukas A, et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

