Vitamin D3 Inhibits Wnt/β-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells
- PMID: 26820714
- PMCID: PMC4880168
- DOI: 10.1210/jc.2015-3555
Vitamin D3 Inhibits Wnt/β-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells
Abstract
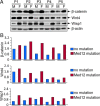
Context: Somatic mutations in the Med12 gene are known to activate Wnt/β-catenin signaling in human uterine fibroids (UFs).
Objective: The objective of the study was to examine the role of vitamin D3 in the modulation of Wnt/β-catenin and mammalian target of rapamycin (mTOR) signaling in human UF cells.
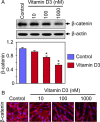
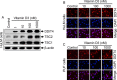
Design: Immortalized human UF cells (HuLM) and human primary UF (PUF) cells were treated with increasing concentrations of vitamin D3 and thereafter analyzed using Western blots and immunocytochemistry.
Main outcome measures: Wnt/β-catenin and mTOR signaling proteins in cultured HuLM and PUF cells were measured.
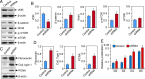
Results: UF tumors with Med12 somatic mutations showed an up-regulation of Wnt4 and β-catenin as compared with adjacent myometrium. Vitamin D3 administration reduced the levels of Wnt4 and β-catenin in both HuLM and PUF cells. Vitamin D3 also reduced the expression/activation of mTOR signaling in both cell types. In contrast, vitamin D3 induced the expression of DNA damaged-induced transcription 4 (an inhibitor of mTOR) and tuberous sclerosis genes (TSC1/2) in a concentration-dependent manner in HuLM cells. Furthermore, we observed a concentration-dependent reduction of Wisp1 (Wnt induced signaling protein 1) and flap endonuclease 1 proteins in HuLM cells. Additionally, abrogation of vitamin D receptor expression (by silencing) in normal myometrial cells induces Wnt4/β-catenin as well as prompts a fibrotic process including an increase in cell proliferation and increased extracellular matrix production. Together these results suggest that vitamin D3 functions as an inhibitor of Wnt4/β-catenin and mTOR signaling pathways, which may play major roles in fibroid pathogenesis.
Conclusions: Vitamin D3 may have utility as a novel long-term therapeutic and/or preventive option for uterine fibroids.
Figures


Similar articles
-
Introduction of Somatic Mutation in MED12 Induces Wnt4/β-Catenin and Disrupts Autophagy in Human Uterine Myometrial Cell.Reprod Sci. 2020 Mar;27(3):823-832. doi: 10.1007/s43032-019-00084-7. Epub 2020 Jan 6. Reprod Sci. 2020. PMID: 32046450 Free PMC article.
-
Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway.Endocrinology. 2017 Mar 1;158(3):592-603. doi: 10.1210/en.2016-1097. Endocrinology. 2017. PMID: 27967206 Free PMC article.
-
EZH2 activates Wnt/β-catenin signaling in human uterine fibroids, which is inhibited by the natural compound methyl jasmonate.F S Sci. 2023 Aug;4(3):239-256. doi: 10.1016/j.xfss.2023.05.003. Epub 2023 May 12. F S Sci. 2023. PMID: 37182601 Free PMC article.
-
Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities.Mol Cell Biochem. 2021 Sep;476(9):3513-3536. doi: 10.1007/s11010-021-04174-6. Epub 2021 May 17. Mol Cell Biochem. 2021. PMID: 33999334 Free PMC article. Review.
-
Role of vitamin D in uterine fibroid biology.Fertil Steril. 2015 Sep;104(3):698-706. doi: 10.1016/j.fertnstert.2015.05.031. Epub 2015 Jun 13. Fertil Steril. 2015. PMID: 26079694 Free PMC article. Review.
Cited by
-
Activation of β-Catenin Signaling and its Crosstalk With Estrogen and Histone Deacetylases in Human Uterine Fibroids.J Clin Endocrinol Metab. 2020 Apr 1;105(4):e1517-35. doi: 10.1210/clinem/dgz227. J Clin Endocrinol Metab. 2020. PMID: 31761932 Free PMC article.
-
Co-existence of benign gynecological tumors with endometriosis in a group of 1,000 women.Oncol Lett. 2018 Feb;15(2):1529-1532. doi: 10.3892/ol.2017.7449. Epub 2017 Nov 20. Oncol Lett. 2018. PMID: 29434846 Free PMC article.
-
The Combination of the CDK4/6 Inhibitor, Palbociclib, With the Vitamin D3 Analog, Inecalcitol, Has Potent In Vitro and In Vivo Anticancer Effects in Hormone-Sensitive Breast Cancer, But Has a More Limited Effect in Triple-Negative Breast Cancer.Front Endocrinol (Lausanne). 2022 Jun 17;13:886238. doi: 10.3389/fendo.2022.886238. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35784555 Free PMC article.
-
Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment.Endocr Rev. 2022 Jul 13;43(4):678-719. doi: 10.1210/endrev/bnab039. Endocr Rev. 2022. PMID: 34741454 Free PMC article. Review.
-
5-aza-2'-deoxycitidine inhibits cell proliferation, extracellular matrix formation and Wnt/β-catenin pathway in human uterine leiomyomas.Reprod Biol Endocrinol. 2021 Jul 8;19(1):106. doi: 10.1186/s12958-021-00790-5. Reprod Biol Endocrinol. 2021. PMID: 34233687 Free PMC article.
References
-
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. - PubMed
-
- Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33(1):59–67. - PubMed
-
- Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):615–626. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

