Axonal transport and secretion of fibrillar forms of α-synuclein, Aβ42 peptide and HTTExon 1
- PMID: 26820848
- PMCID: PMC4789229
- DOI: 10.1007/s00401-016-1538-0
Axonal transport and secretion of fibrillar forms of α-synuclein, Aβ42 peptide and HTTExon 1
Abstract
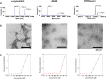
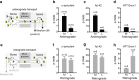
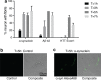
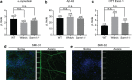
Accruing evidence suggests that prion-like behavior of fibrillar forms of α-synuclein, β-amyloid peptide and mutant huntingtin are responsible for the spread of the lesions that characterize Parkinson disease, Alzheimer disease and Huntington disease, respectively. It is unknown whether these distinct protein assemblies are transported within and between neurons by similar or distinct mechanisms. It is also unclear if neuronal death or injury is required for neuron-to-neuron transfer. To address these questions, we used mouse primary cortical neurons grown in microfluidic devices to measure the amounts of α-synuclein, Aβ42 and HTTExon1 fibrils transported by axons in both directions (anterograde and retrograde), as well as to examine the mechanism of their release from axons after anterograde transport. We observed that the three fibrils were transported in both anterograde and retrograde directions but with strikingly different efficiencies. The amount of Aβ42 fibrils transported was ten times higher than that of the other two fibrils. HTTExon1 was efficiently transported in the retrograde direction but only marginally in the anterograde direction. Finally, using neurons from two distinct mutant mouse strains whose axons are highly resistant to neurodegeneration (Wld(S) and Sarm1(-/-)), we found that the three different fibrils were secreted by axons after anterograde transport, in the absence of axonal lysis, indicating that trans-neuronal spread can occur in intact healthy neurons. In summary, fibrils of α-synuclein, Aβ42 and HTTExon1 are all transported in axons but in directions and amounts that are specific of each fibril. After anterograde transport, the three fibrils were secreted in the medium in the absence of axon lysis. Continuous secretion could play an important role in the spread of pathology between neurons but may be amenable to pharmacological intervention.
Keywords: Axonal transport; Aβ42; HTTExon1; Secretion; α-Synuclein.
Figures
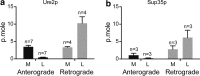
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

