Recurrent Moderate Hypoglycemia Suppresses Brain-Derived Neurotrophic Factor Expression in the Prefrontal Cortex and Impairs Sensorimotor Gating in the Posthypoglycemic Period in Young Rats
- PMID: 26820887
- PMCID: PMC4747640
- DOI: 10.1159/000442878
Recurrent Moderate Hypoglycemia Suppresses Brain-Derived Neurotrophic Factor Expression in the Prefrontal Cortex and Impairs Sensorimotor Gating in the Posthypoglycemic Period in Young Rats
Abstract
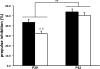
Recurrent hypoglycemia is common in infants and children. In developing rat models, recurrent moderate hypoglycemia leads to neuronal injury in the medial prefrontal cortex. To understand the effects beyond neuronal injury, 3-week-old male rats were subjected to 5 episodes of moderate hypoglycemia (blood glucose concentration, approx. 30 mg/dl for 90 min) once daily from postnatal day 24 to 28. Neuronal injury was determined using Fluoro-Jade B histochemistry on postnatal day 29. The effects on brain-derived neurotrophic factor (BDNF) and its cognate receptor, tyrosine kinase receptor B (TrkB) expression, which is critical for prefrontal cortex development, were determined on postnatal day 29 and at adulthood. The effects on prefrontal cortex-mediated function were determined by assessing the prepulse inhibition of the acoustic startle reflex on postnatal day 29 and 2 weeks later, and by testing for fear-potentiated startle at adulthood. Recurrent hypoglycemia led to neuronal injury confined primarily to the medial prefrontal cortex. BDNF/TrkB expression in the prefrontal cortex was suppressed on postnatal day 29 and was accompanied by lower prepulse inhibition, suggesting impaired sensorimotor gating. Following the cessation of recurrent hypoglycemia, the prepulse inhibition had recovered at 2 weeks. BDNF/TrkB expression in the prefrontal cortex had normalized and fear-potentiated startle was intact at adulthood. Recurrent moderate hypoglycemia during development has significant adverse effects on the prefrontal cortex in the posthypoglycemic period.
© 2016 S. Karger AG, Basel.
Conflict of interest statement
None of the authors have conflict of interest to declare.
Figures


Similar articles
-
Prefrontal D1 and ventral hippocampal N-methyl-D-aspartate regulation of startle gating in rats.Neuroscience. 2005;135(2):385-94. doi: 10.1016/j.neuroscience.2005.06.054. Neuroscience. 2005. PMID: 16125865 Free PMC article.
-
Chronic clozapine treatment improves the alterations of prepulse inhibition and BDNF mRNA expression in the medial prefrontal cortex that are induced by adolescent social isolation.Behav Pharmacol. 2019 Jun;30(4):311-319. doi: 10.1097/FBP.0000000000000419. Behav Pharmacol. 2019. PMID: 30157036
-
Altered Acoustic Startle Reflex, Prepulse Inhibition, and Peripheral Brain-Derived Neurotrophic Factor in Morphine Self-Administered Rats.Int J Neuropsychopharmacol. 2017 May 1;20(5):383-391. doi: 10.1093/ijnp/pyw107. Int J Neuropsychopharmacol. 2017. PMID: 27927738 Free PMC article.
-
Neuronal coding of auditory sensorimotor gating in medial prefrontal cortex.Behav Brain Res. 2017 May 30;326:200-208. doi: 10.1016/j.bbr.2017.03.004. Epub 2017 Mar 8. Behav Brain Res. 2017. PMID: 28284946
-
Deficient sensorimotor gating after 6-hydroxydopamine lesion of the rat medial prefrontal cortex is reversed by haloperidol.Eur J Neurosci. 1994 Dec 1;6(12):1837-45. doi: 10.1111/j.1460-9568.1994.tb00576.x. Eur J Neurosci. 1994. PMID: 7704295
Cited by
-
Early-Life Iron Deficiency Alters Glucose Transporter-1 Expression in the Adult Rodent Hippocampus.J Nutr. 2019 Sep 1;149(9):1660-1666. doi: 10.1093/jn/nxz100. J Nutr. 2019. PMID: 31162576 Free PMC article.
-
Analysis of risk factors of neonatal hypoglycemia and its correlation with blood glucose control of gestational diabetes mellitus: A retrospective study.Medicine (Baltimore). 2023 Sep 1;102(35):e34619. doi: 10.1097/MD.0000000000034619. Medicine (Baltimore). 2023. PMID: 37657063 Free PMC article.
-
Recurrent moderate hypoglycemia exacerbates oxidative damage and neuronal death leading to cognitive dysfunction after the hypoglycemic coma.J Cereb Blood Flow Metab. 2019 May;39(5):808-821. doi: 10.1177/0271678X17733640. Epub 2017 Oct 19. J Cereb Blood Flow Metab. 2019. PMID: 29047291 Free PMC article.
References
-
- Rao R, Hershey T. The impact of hypoglycemia on the developing brain. In: Seaquist ER, Robertson PR, editors. Translational endocrinology and metabolism: Hypoglycemia in diabetes update. 4. Vol. 3. Chevy Chase, MD: Endocrine Society; 2012. pp. 137–159.
-
- Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. - PubMed
-
- Blasetti A, Chiuri RM, Tocco AM, Di Giulio C, Mattei PA, Ballone E, Chiarelli F, Verrotti A. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: A meta-analysis. J Child Neurol. 2011;26:1383–1391. - PubMed
-
- Reich JN, Kaspar JC, Puczynski MS, Puczynski S, Cleland JW, Dell’Angela K, Emanuele MA. Effect of a hypoglycemic episode on neuropsychological functioning in diabetic children. J Clin Exp Neuropsychol. 1990;12:613–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

