Design of a MCoTI-Based Cyclotide with Angiotensin (1-7)-Like Activity
- PMID: 26821010
- PMCID: PMC4795166
- DOI: 10.3390/molecules21020152
Design of a MCoTI-Based Cyclotide with Angiotensin (1-7)-Like Activity
Abstract
We report for the first time the design and synthesis of a novel cyclotide able to activate the unique receptor of angiotensin (1-7) (AT1-7), the MAS1 receptor. This was accomplished by grafting an AT1-7 peptide analog onto loop 6 of cyclotide MCoTI-I using isopeptide bonds to preserve the α-amino and C-terminal carboxylate groups of AT1-7, which are required for activity. The resulting cyclotide construct was able to adopt a cyclotide-like conformation and showed similar activity to that of AT1-7. This cyclotide also showed high stability in human serum thereby providing a promising lead compound for the design of a novel type of peptide-based in the treatment of cancer and myocardial infarction.
Keywords: MAS1 receptor; angiotensin; cyclotide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


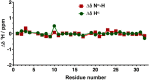
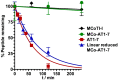
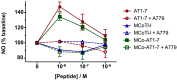
References
-
- Menon J., Soto-Pantoja D.R., Callahan M.F., Cline J.M., Ferrario C.M., Tallant E.A., Gallagher P.E. Angiotensin-(1–7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in Cyclooxygenase-2. Cancer Res. 2007;67:2809–2815. doi: 10.1158/0008-5472.CAN-06-3614. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

