Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae)
- PMID: 26821032
- PMCID: PMC4771978
- DOI: 10.3390/md14020020
Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae)
Abstract
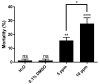
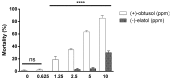
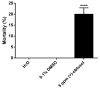
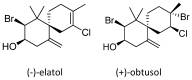
Dengue is considered a serious public health problem in many tropical regions of the world including Brazil. At the moment, there is no viable alternative to reduce dengue infections other than controlling the insect vector, Aedes aegypti Linnaeus. In the continuing search for new sources of chemicals targeted at vector control, natural products are a promising alternative to synthetic pesticides. In our work, we investigated the toxicity of a bioactive compound extracted from the red alga Laurencia dendroidea J. Agardh. The initial results demonstrated that crude extracts, at a concentration of 5 ppm, caused pronounced mortality of second instar A. aegypti larvae. Two molecules, identified as (-)-elatol and (+)-obtusol were subsequently isolated from crude extract and further evaluated. Assays with (-)-elatol showed moderate larvicidal activity, whereas (+)-obtusol presented higher toxic activity than (-)-elatol, with a LC50 value of 3.5 ppm. Histological analysis of the larvae exposed to (+)-obtusol revealed damage to the intestinal epithelium. Moreover, (+)-obtusol-treated larvae incubated with 2 µM CM-H₂DCFDA showed the presence of reactive oxygen species, leading us to suggest that epithelial damage might be related to redox imbalance. These results demonstrate the potential of (+)-obtusol as a larvicide for use against A. aegypti and the possible mode of action of this compound.
Keywords: (+)-obtusol; (−)-elatol; Aedes aegypti; Laurencia dendroidea; larvicide; oxidative stress; sesquiterpenes.
Figures





Similar articles
-
Cytotoxic activity of halogenated sesquiterpenes from Laurencia dendroidea.Phytother Res. 2018 Jun;32(6):1119-1125. doi: 10.1002/ptr.6052. Epub 2018 Feb 26. Phytother Res. 2018. PMID: 29480520
-
Nitric Oxide Production Inhibition and Anti-Mycobacterial Activity of Extracts and Halogenated Sesquiterpenes from the Brazilian Red Alga Laurencia Dendroidea J. Agardh.Pharmacogn Mag. 2015 Oct;11(Suppl 4):S611-8. doi: 10.4103/0973-1296.172972. Pharmacogn Mag. 2015. PMID: 27013803 Free PMC article.
-
Antileishmanial sesquiterpenes from the Brazilian red alga Laurencia dendroidea.Planta Med. 2011 May;77(7):733-5. doi: 10.1055/s-0030-1250526. Epub 2010 Nov 5. Planta Med. 2011. PMID: 21058243
-
Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae.Acta Trop. 2020 Dec;212:105705. doi: 10.1016/j.actatropica.2020.105705. Epub 2020 Sep 19. Acta Trop. 2020. PMID: 32956639 Review.
-
Larvicidal activity of terpenes and their derivatives against Aedes aegypti: a systematic review and meta-analysis.Environ Sci Pollut Res Int. 2024 Dec;31(56):64703-64718. doi: 10.1007/s11356-024-35479-w. Epub 2024 Nov 16. Environ Sci Pollut Res Int. 2024. PMID: 39549195
Cited by
-
Algal-Derived Halogenated Sesquiterpenes from Laurencia dendroidea as Lead Compounds in Schistosomiasis Environmental Control.Mar Drugs. 2022 Jan 29;20(2):111. doi: 10.3390/md20020111. Mar Drugs. 2022. PMID: 35200640 Free PMC article.
-
Screening and evaluation of different algal extracts and prospects for controlling the disease vector mosquito Culex pipiens L.Saudi J Biol Sci. 2022 Feb;29(2):933-940. doi: 10.1016/j.sjbs.2021.10.009. Epub 2021 Oct 12. Saudi J Biol Sci. 2022. PMID: 35197761 Free PMC article.
-
Characterization of some selected macroalgae extracts and assessment of their insecticidal and genotoxicity in Culex pipiens L. mosquito larvae.Sci Rep. 2025 Jan 21;15(1):2655. doi: 10.1038/s41598-025-86347-7. Sci Rep. 2025. PMID: 39838058 Free PMC article.
-
Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: a review.Environ Chem Lett. 2023;21(1):97-152. doi: 10.1007/s10311-022-01520-y. Epub 2022 Oct 8. Environ Chem Lett. 2023. PMID: 36245550 Free PMC article. Review.
-
Phytochemical Investigation of Three Cystoseira Species and Their Larvicidal Activity Supported with In Silico Studies.Mar Drugs. 2023 Feb 10;21(2):117. doi: 10.3390/md21020117. Mar Drugs. 2023. PMID: 36827158 Free PMC article.
References
-
- World Health Organization (WHO) Dengue and Severe Dengue. [(accessed on 5 November 2015)]. Updated May 2015. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/
-
- Marchette N.J., Garcia R., Rudnick A. Isolation of Zika Virus from Aedes aegypti Mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969;18:411–415. - PubMed
-
- Ranson H., Burhani J., Lumjuan N., Black I.V.W.C. Insecticide Resistance in Dengue Vectors. [(accessed on 24 November 2014)];TropIKA.net.(online) 2010 1:0–0. ISSN 2078-8606. Available online: http://journal.tropika.net/scielo.php?script=sci_arttext.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

