X-linked inhibitor of apoptosis protein mediates tumor cell resistance to antibody-dependent cellular cytotoxicity
- PMID: 26821068
- PMCID: PMC4816185
- DOI: 10.1038/cddis.2015.412
X-linked inhibitor of apoptosis protein mediates tumor cell resistance to antibody-dependent cellular cytotoxicity
Abstract
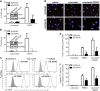
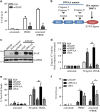
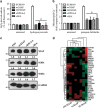
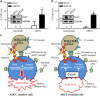
Inflammatory breast cancer (IBC) is the deadliest, distinct subtype of breast cancer. High expression of epidermal growth factor receptors [EGFR or human epidermal growth factor receptor 2 (HER2)] in IBC tumors has prompted trials of anti-EGFR/HER2 monoclonal antibodies to inhibit oncogenic signaling; however, de novo and acquired therapeutic resistance is common. Another critical function of these antibodies is to mediate antibody-dependent cellular cytotoxicity (ADCC), which enables immune effector cells to engage tumors and deliver granzymes, activating executioner caspases. We hypothesized that high expression of anti-apoptotic molecules in tumors would render them resistant to ADCC. Herein, we demonstrate that the most potent caspase inhibitor, X-linked inhibitor of apoptosis protein (XIAP), overexpressed in IBC, drives resistance to ADCC mediated by cetuximab (anti-EGFR) and trastuzumab (anti-HER2). Overexpression of XIAP in parental IBC cell lines enhances resistance to ADCC; conversely, targeted downregulation of XIAP in ADCC-resistant IBC cells renders them sensitive. As hypothesized, this ADCC resistance is in part a result of the ability of XIAP to inhibit caspase activity; however, we also unexpectedly found that resistance was dependent on XIAP-mediated, caspase-independent suppression of reactive oxygen species (ROS) accumulation, which otherwise occurs during ADCC. Transcriptome analysis supported these observations by revealing modulation of genes involved in immunosuppression and oxidative stress response in XIAP-overexpressing, ADCC-resistant cells. We conclude that XIAP is a critical modulator of ADCC responsiveness, operating through both caspase-dependent and -independent mechanisms. These results suggest that strategies targeting the effects of XIAP on caspase activation and ROS suppression have the potential to enhance the activity of monoclonal antibody-based immunotherapy.
Figures


References
-
- Robertson FM, Bondy M, Yang W, Yamauchi H, Wiggins S, Kamrudin S et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin 2010; 60: 351–375. - PubMed
-
- Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA et al. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol 1997; 40: 321–329. - PubMed
-
- Prost S, Le MG, Douc-Rasy S, Ahomadegbe JC, Spielmann M, Guerin M et al. Association of c-erbB2-gene amplification with poor prognosis in non-inflammatory breast carcinomas but not in carcinomas of the inflammatory type. Int J Cancer 1994; 58: 763–768. - PubMed
-
- Van Laere SJ, Van der Auwera I, Van den Eynden GG, van Dam P, Van Marck EA, Vermeulen PB et al. NF-kappaB activation in inflammatory breast cancer is associated with oestrogen receptor downregulation, secondary to EGFR and/or ErbB2 overexpression and MAPK hyperactivation. Br J Cancer 2007; 97: 659–669. - PMC - PubMed
-
- Cabioglu N, Gong Y, Islam R, Broglio KR, Sneige N, Sahin A et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol 2007; 18: 1021–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

