Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review
- PMID: 26821255
- PMCID: PMC4731557
- DOI: 10.1371/journal.pone.0147170
Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review
Abstract
The Acute Respiratory Distress Syndrome (ARDS) is a devastating clinical condition that is associated with a 30-40% risk of death, and significant long term morbidity for those who survive. Mesenchymal stromal cells (MSC) have emerged as a potential novel treatment as in pre-clinical models they have been shown to modulate inflammation (a major pathophysiological hallmark of ARDS) while enhancing bacterial clearance and reducing organ injury and death. A systematic search of MEDLINE, EMBASE, BIOSIS and Web of Science was performed to identify pre-clinical studies that examined the efficacy MSCs as compared to diseased controls for the treatment of Acute Lung Injury (ALI) (the pre-clinical correlate of human ARDS) on mortality, a clinically relevant outcome. We assessed study quality and pooled results using random effect meta-analysis. A total of 54 publications met our inclusion criteria of which 17 (21 experiments) reported mortality and were included in the meta-analysis. Treatment with MSCs, as compared to controls, significantly decreased the overall odds of death in animals with ALI (Odds Ratio 0.24, 95% Confidence Interval 0.18-0.34, I2 8%). Efficacy was maintained across different types of animal models and means of ALI induction; MSC origin, source, route of administration and preparation; and the clinical relevance of the model (timing of MSC administration, administration of fluids and or antibiotics). Reporting of standard MSC characterization for experiments that used human MSCs and risks of bias was generally poor, and although not statistically significant, a funnel plot analysis for overall mortality suggested the presence of publication bias. The results from our meta-analysis support that MSCs substantially reduce the odds of death in animal models of ALI but important reporting elements were sub optimal and limit the strength of our conclusions.
Conflict of interest statement
Figures
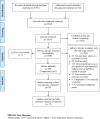

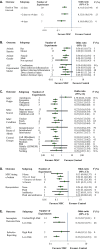
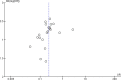
References
-
- Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003; 348(8):683–693. - PubMed
-
- Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354(24):2564–2575. - PubMed
-
- Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA 2006; 296(14):1731–1732. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

