Very-low and low-density lipoproteins induce neutral lipid accumulation and impair migration in monocyte subsets
- PMID: 26821597
- PMCID: PMC4731823
- DOI: 10.1038/srep20038
Very-low and low-density lipoproteins induce neutral lipid accumulation and impair migration in monocyte subsets
Abstract
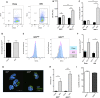
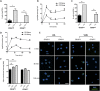
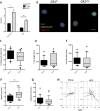
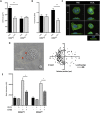
Blood monocytes are heterogeneous effector cells of the innate immune system. In circulation these cells are constantly in contact with lipid-rich lipoproteins, yet this interaction is poorly characterised. Our aim was to examine the functional effect of hyperlipidaemia on blood monocytes. In the Ldlr(-/-) mouse monocytes rapidly accumulate cytoplasmic neutral lipid vesicles during hyperlipidaemia. Functional analysis in vivo revealed impaired monocyte chemotaxis towards peritonitis following high fat diet due to retention of monocytes in the greater omentum. In vitro assays using human monocytes confirmed neutral lipid vesicle accumulation after exposure to LDL or VLDL. Neutral lipid accumulation did not inhibit phagocytosis, endothelial adhesion, intravascular crawling and transmigration. However, lipid loading led to a migratory defect towards C5a and disruption of cytoskeletal rearrangement, including an inhibition of RHOA signaling. These data demonstrate distinct effects of hyperlipidaemia on the chemotaxis and cytoskeletal regulation of monocyte subpopulations. These data emphasise the functional consequences of blood monocyte lipid accumulation and reveal important implications for treating inflammation, infection and atherosclerosis in the context of dyslipidaemia.
Figures


References
-
- Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell. Immunol. 289, 135–139 (2014). - PubMed
-
- Wong K. L. et al.. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118, e16–e31 (2011). - PubMed
-
- Zawada A. M. et al.. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 118, e50–61 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

