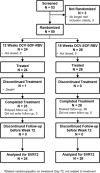
Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+)
- PMID: 26822022
- PMCID: PMC5069621
- DOI: 10.1002/hep.28473
Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+)
Abstract
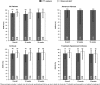
Patients with hepatitis C virus (HCV) genotype 3 infection, especially those with advanced liver disease, are a challenging population in urgent need of optimally effective therapies. The combination of daclatasvir (DCV; pangenotypic nonstructural protein 5A inhibitor) and sofosbuvir (SOF; nucleotide nonstructural protein 5B inhibitor) for 12 weeks previously showed high efficacy (96%) in noncirrhotic genotype 3 infection. The phase III ALLY-3+ study (N = 50) evaluated DCV-SOF with ribavirin (RBV) in treatment-naïve (n = 13) or treatment-experienced (n = 37) genotype 3-infected patients with advanced fibrosis (n = 14) or compensated cirrhosis (n = 36). Patients were randomized 1:1 to receive open-label DCV-SOF (60 + 400 mg daily) with weight-based RBV for 12 or 16 weeks. The primary endpoint was sustained virological response at post-treatment week 12 (SVR12). SVR12 (intention-to-treat) was 90% overall (45 of 50): 88% (21 of 24) in the 12-week (91% observed) and 92% (24 of 26) in the 16-week group. All patients with advanced fibrosis achieved SVR12. SVR12 in patients with cirrhosis was 86% overall (31 of 36): 83% (15 of 18) in the 12-week (88% observed) and 89% (16 of 18) in the 16-week group; for treatment-experienced patients with cirrhosis, these values were 87% (26 of 30), 88% (14 of 16; 93% observed), and 86% (12 of 14), respectively. One patient (12-week group) did not enter post-treatment follow-up (death unrelated to treatment). There were 4 relapses (2 per group) and no virological breakthroughs. The most common adverse events (AEs) were insomnia, fatigue, and headache. There were no discontinuations for AEs and no treatment-related serious AEs.
Conclusion: The all-oral regimen of DCV-SOF-RBV was well tolerated and resulted in high and similar SVR12 after 12 or 16 weeks of treatment among genotype 3-infected patients with advanced liver disease, irrespective of past HCV treatment experience.
© 2016 The Authors. HEPATOLOGY published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures
Similar articles
-
High Efficacy and Safety of Flat-Dose Ribavirin Plus Sofosbuvir/Daclatasvir in Genotype 3 Cirrhotic Patients.Gut Liver. 2020 May 15;14(3):357-367. doi: 10.5009/gnl18269. Gut Liver. 2020. PMID: 30970444 Free PMC article.
-
Comparative efficacy of sofosbuvir-ribavirin versus sofosbuvir-daclatasvir for treatment of chronic hepatitis C in an area with limited NS5A inhibitor availability.Indian J Gastroenterol. 2018 Nov;37(6):520-525. doi: 10.1007/s12664-018-0921-2. Epub 2019 Jan 12. Indian J Gastroenterol. 2018. PMID: 30637537
-
Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort.Gut. 2016 Nov;65(11):1861-1870. doi: 10.1136/gutjnl-2016-312444. Epub 2016 Sep 7. Gut. 2016. PMID: 27605539 Free PMC article.
-
Daclatasvir and Sofosbuvir Versus Sofosbuvir and Ribavirin in Patients with Chronic Hepatitis C Coinfected with HIV: A Matching-adjusted Indirect Comparison.Clin Ther. 2016 Feb;38(2):404-12. doi: 10.1016/j.clinthera.2015.12.017. Epub 2016 Feb 3. Clin Ther. 2016. PMID: 26839044
-
Daclatasvir: A NS5A Replication Complex Inhibitor for Hepatitis C Infection.Ann Pharmacother. 2016 Jan;50(1):39-46. doi: 10.1177/1060028015610342. Epub 2015 Oct 20. Ann Pharmacother. 2016. PMID: 26486762 Review.
Cited by
-
Successful treatment of hepatitis C genotype 4 using sofosbuvir, daclatasvir, simeprevir and ribavirin in Egyptian patients with direct-acting antiviral agent treatment failure.Clin Exp Hepatol. 2022 Mar;8(1):36-41. doi: 10.5114/ceh.2022.114246. Epub 2022 Mar 23. Clin Exp Hepatol. 2022. PMID: 35415259 Free PMC article.
-
Polymorphisms in the hepatitis C virus core and its association with development of hepatocellular carcinoma.J Biosci. 2017 Sep;42(3):509-521. doi: 10.1007/s12038-017-9695-4. J Biosci. 2017. PMID: 29358564 Review.
-
All-oral direct antiviral treatment for hepatitis C chronic infection in a real-life cohort: The role of cirrhosis and comorbidities in treatment response.PLoS One. 2018 Jul 10;13(7):e0199941. doi: 10.1371/journal.pone.0199941. eCollection 2018. PLoS One. 2018. PMID: 29990371 Free PMC article.
-
Efficacy of Interferon-Free Therapies for Chronic Hepatitis C: A Systematic Review of All Randomized Clinical Trials.Clin Drug Investig. 2017 Jul;37(7):635-646. doi: 10.1007/s40261-017-0521-4. Clin Drug Investig. 2017. PMID: 28409482
-
Hepatitis C virus genotype 3: clinical features, current and emerging viral inhibitors, future challenges.Ann Gastroenterol. 2018 Sep-Oct;31(5):541-551. doi: 10.20524/aog.2018.0281. Epub 2018 Jun 4. Ann Gastroenterol. 2018. PMID: 30174390 Free PMC article. Review.
References
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558‐567. - PubMed
-
- Stamouli M, Panagiotou I, Kairis D, Michopoulou A, Skliris A, Totos G. Genotype distribution in chronic hepatitis C patients in Greece. Clin Lab 2012;58:173‐176. - PubMed
-
- Chlabicz S, Flisiak R, Kowalczuk O, Grzeszczuk A, Pytel‐Krolczuk B, Prokopowicz D, et al. Changing HCV genotypes distribution in Poland‐relation to source and time of infection. J Clin Virol 2008;42:156‐159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous