Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde
- PMID: 26822067
- PMCID: PMC4937616
- DOI: 10.1136/thoraxjnl-2015-207895
Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde
Abstract
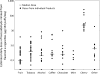
Many non-cigarette tobacco products, including e-cigarettes, contain various flavourings, such as fruit flavours. Although many flavourings used in e-cigarettes are generally recognised as safe when used in food products, concerns have been raised about the potential inhalation toxicity of these chemicals. Benzaldehyde, which is a key ingredient in natural fruit flavours, has been shown to cause irritation of respiratory airways in animal and occupational exposure studies. Given the potential inhalation toxicity of this compound, we measured benzaldehyde in aerosol generated in a laboratory setting from flavoured e-cigarettes purchased online and detected benzaldehyde in 108 out of 145 products. The highest levels of benzaldehyde were detected in cherry-flavoured products. The benzaldehyde doses inhaled with 30 puffs from flavoured e-cigarettes were often higher than doses inhaled from a conventional cigarette. Levels in cherry-flavoured products were >1000 times lower than doses inhaled in the workplace. While e-cigarettes seem to be a promising harm reduction tool for smokers, findings indicate that using these products could result in repeated inhalation of benzaldehyde, with long-term users risking regular exposure to the substance. Given the uncertainty surrounding adverse health effects stemming from long-term inhalation of flavouring ingredients such as benzaldehyde, clinicians need to be aware of this emerging risk and ask their patients about use of flavoured e-cigarettes.
Keywords: Drug induced Lung Disease; Inhaler devices; Tobacco and the lung; Tobacco control.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Conflict of interest statement
References
-
- Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. Hyattsville, MD: National Center for Health Statistics; Oct, 2015. (NCHS Data Brief, no 217). - PubMed
-
- Overview of the Family Smoking Prevention and Tobacco Control Act, June, 2009. Retrieved from http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulator....
-
- The MAK Collection for Occupational Health and Safety. Wiley-VCH Verlag GmbH & Co. KGaA; 2012. Benzaldehyde [MAK Value Documentation, 2002] pp. 14–36. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical