Heterogeneous dynamics, robustness/fragility trade-offs, and the eradication of the macroparasitic disease, lymphatic filariasis
- PMID: 26822124
- PMCID: PMC4731922
- DOI: 10.1186/s12916-016-0557-y
Heterogeneous dynamics, robustness/fragility trade-offs, and the eradication of the macroparasitic disease, lymphatic filariasis
Abstract
Background: The current WHO-led initiative to eradicate the macroparasitic disease, lymphatic filariasis (LF), based on single-dose annual mass drug administration (MDA) represents one of the largest health programs devised to reduce the burden of tropical diseases. However, despite the advances made in instituting large-scale MDA programs in affected countries, a challenge to meeting the goal of global eradication is the heterogeneous transmission of LF across endemic regions, and the impact that such complexity may have on the effort required to interrupt transmission in all socioecological settings.
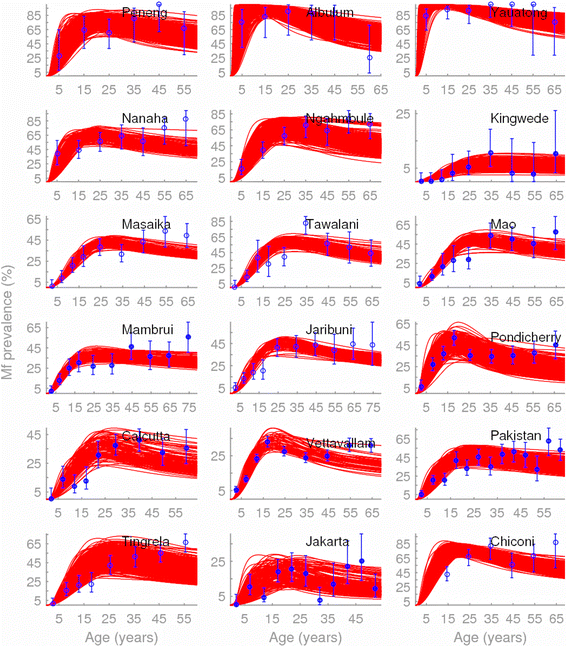
Methods: Here, we apply a Bayesian computer simulation procedure to fit transmission models of LF to field data assembled from 18 sites across the major LF endemic regions of Africa, Asia and Papua New Guinea, reflecting different ecological and vector characteristics, to investigate the impacts and implications of transmission heterogeneity and complexity on filarial infection dynamics, system robustness and control.
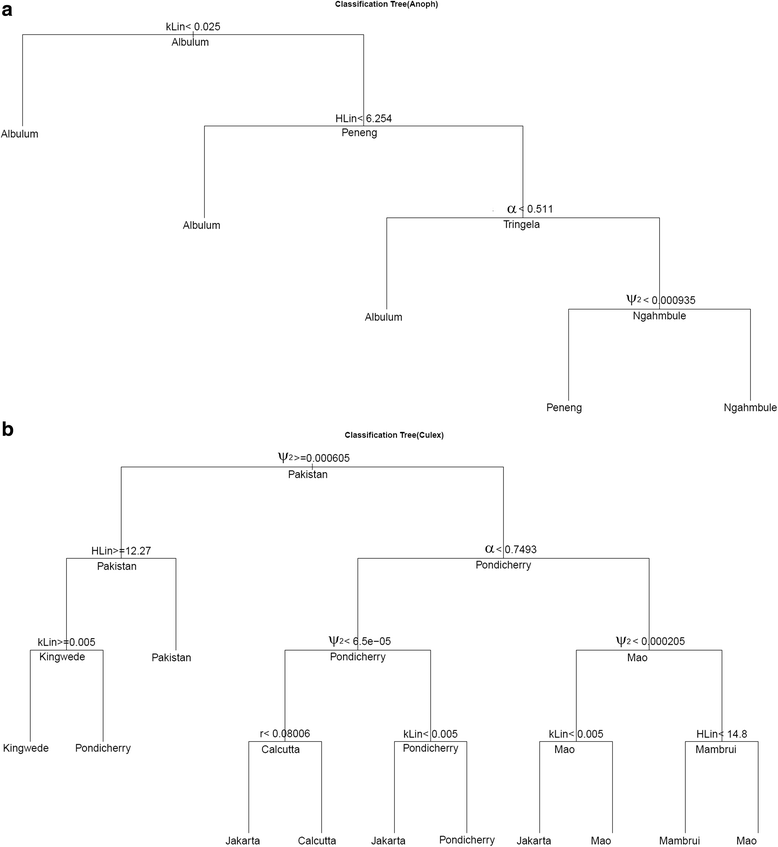
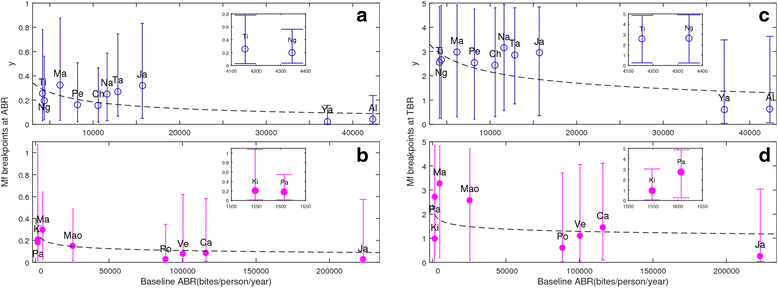
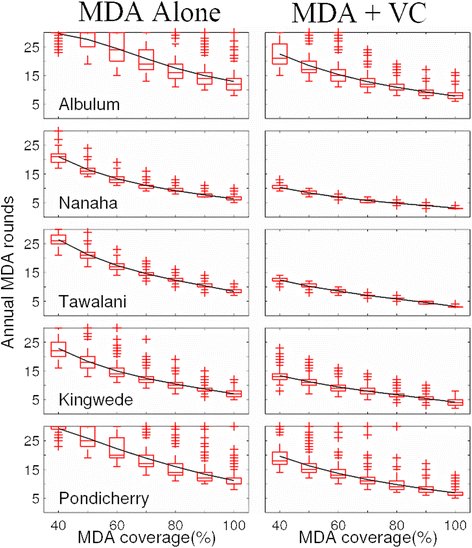
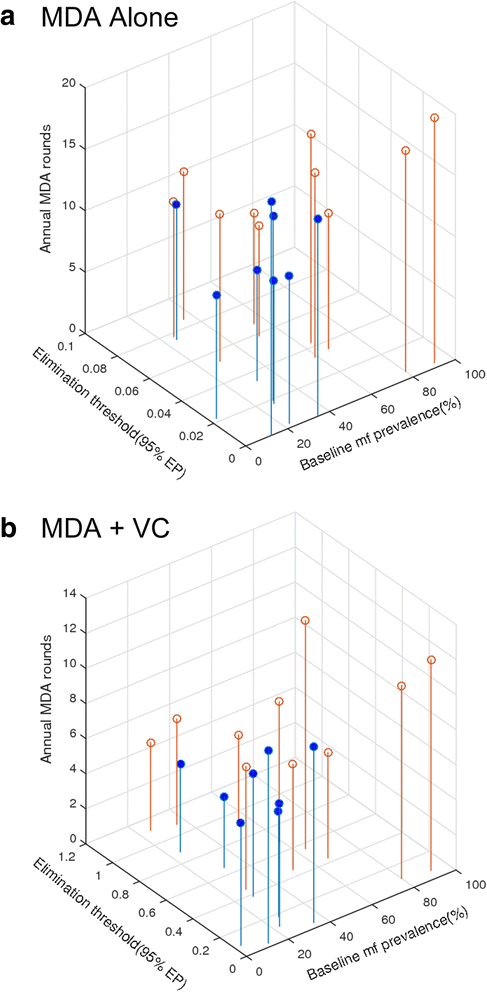
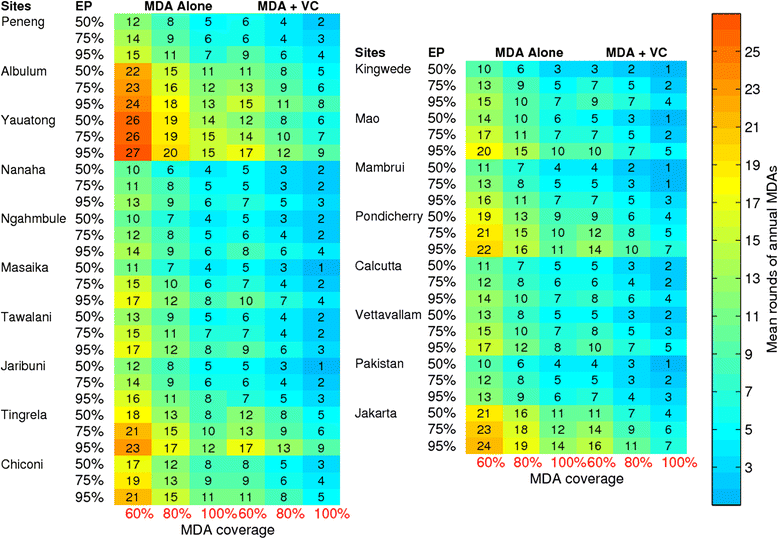
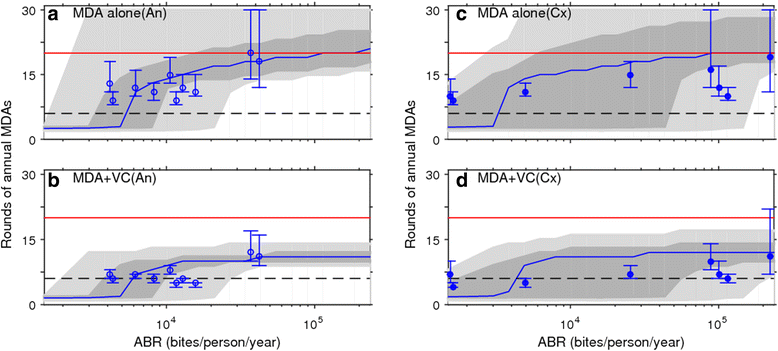
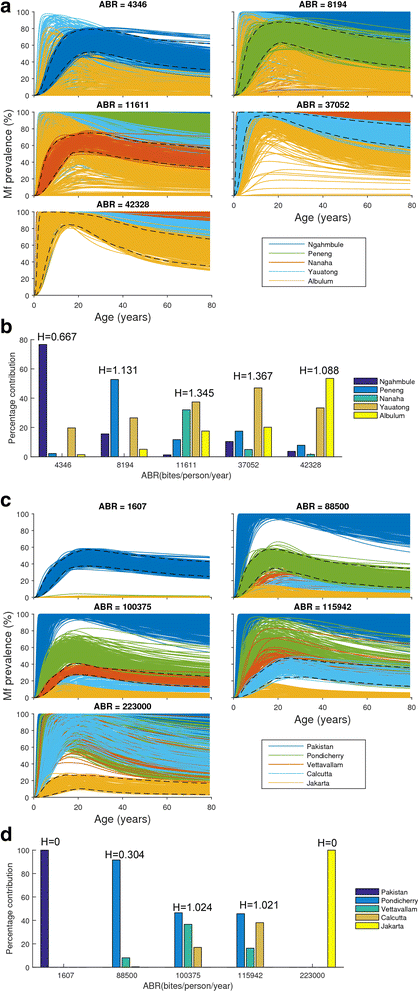
Results: We find firstly that LF elimination thresholds varied significantly between the 18 study communities owing to site variations in transmission and initial ecological parameters. We highlight how this variation in thresholds lead to the need for applying variable durations of interventions across endemic communities for achieving LF elimination; however, a major new result is the finding that filarial population responses to interventions ultimately reflect outcomes of interplays between dynamics and the biological architectures and processes that generate robustness/fragility trade-offs in parasite transmission. Intervention simulations carried out in this study further show how understanding these factors is also key to the design of options that would effectively eliminate LF from all settings. In this regard, we find how including vector control into MDA programs may not only offer a countermeasure that will reliably increase system fragility globally across all settings and hence provide a control option robust to differential locality-specific transmission dynamics, but by simultaneously reducing transmission regime variability also permit more reliable macroscopic predictions of intervention effects.
Conclusions: Our results imply that a new approach, combining adaptive modelling of parasite transmission with the use of biological robustness as a design principle, is required if we are to both enhance understanding of complex parasitic infections and delineate options to facilitate their elimination effectively.
Figures
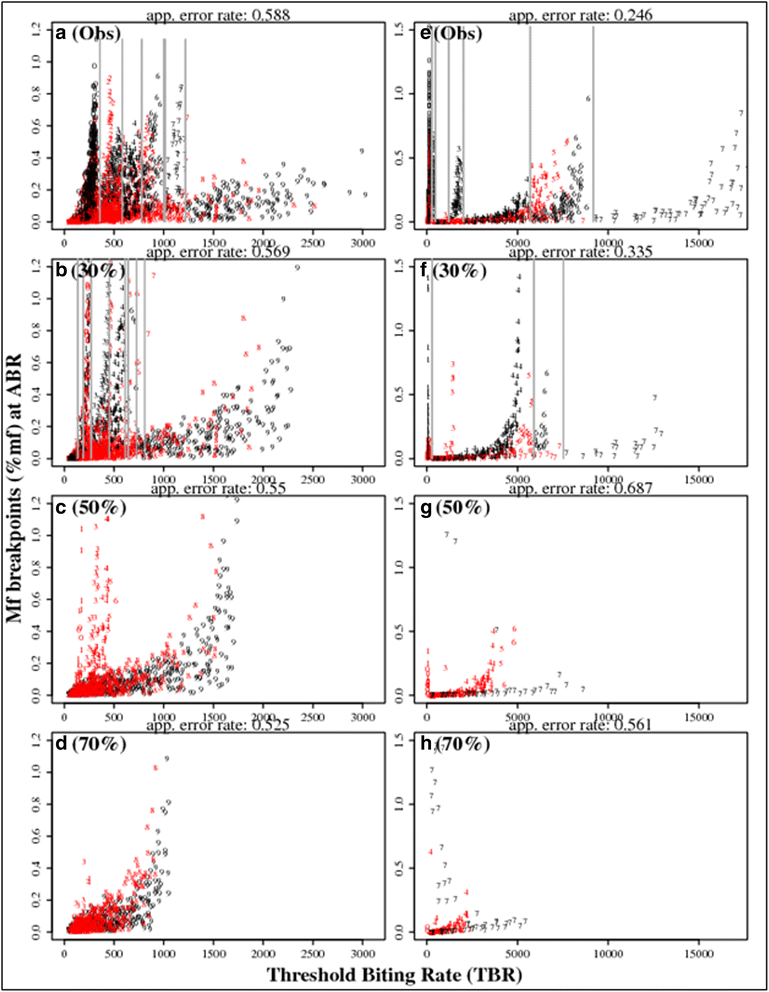
Similar articles
-
Continental-scale, data-driven predictive assessment of eliminating the vector-borne disease, lymphatic filariasis, in sub-Saharan Africa by 2020.BMC Med. 2017 Sep 27;15(1):176. doi: 10.1186/s12916-017-0933-2. BMC Med. 2017. PMID: 28950862 Free PMC article.
-
Geographic and ecologic heterogeneity in elimination thresholds for the major vector-borne helminthic disease, lymphatic filariasis.BMC Biol. 2010 Mar 17;8:22. doi: 10.1186/1741-7007-8-22. BMC Biol. 2010. PMID: 20236528 Free PMC article.
-
Bayesian calibration of simulation models for supporting management of the elimination of the macroparasitic disease, Lymphatic Filariasis.Parasit Vectors. 2015 Oct 22;8:522. doi: 10.1186/s13071-015-1132-7. Parasit Vectors. 2015. PMID: 26490350 Free PMC article.
-
Lymphatic filariasis in Asia: a systematic review and meta-analysis.Parasitol Res. 2021 Feb;120(2):411-422. doi: 10.1007/s00436-020-06991-y. Epub 2021 Jan 8. Parasitol Res. 2021. PMID: 33415391 Free PMC article.
-
Lessons from lymphatic filariasis elimination and the challenges of post-elimination surveillance in China.Infect Dis Poverty. 2019 Aug 7;8(1):66. doi: 10.1186/s40249-019-0578-9. Infect Dis Poverty. 2019. PMID: 31387644 Free PMC article. Review.
Cited by
-
Comprehensive Assessment of a Hotspot with Persistent Bancroftian Filariasis in Coastal Sri Lanka.Am J Trop Med Hyg. 2018 Sep;99(3):735-742. doi: 10.4269/ajtmh.18-0169. Epub 2018 Jul 12. Am J Trop Med Hyg. 2018. PMID: 30014812 Free PMC article.
-
Predicting lymphatic filariasis elimination in data-limited settings: A reconstructive computational framework for combining data generation and model discovery.PLoS Comput Biol. 2020 Jul 21;16(7):e1007506. doi: 10.1371/journal.pcbi.1007506. eCollection 2020 Jul. PLoS Comput Biol. 2020. PMID: 32692741 Free PMC article.
-
Continental-scale, data-driven predictive assessment of eliminating the vector-borne disease, lymphatic filariasis, in sub-Saharan Africa by 2020.BMC Med. 2017 Sep 27;15(1):176. doi: 10.1186/s12916-017-0933-2. BMC Med. 2017. PMID: 28950862 Free PMC article.
-
Assessing endgame strategies for the elimination of lymphatic filariasis: A model-based evaluation of the impact of DEC-medicated salt.Sci Rep. 2017 Aug 7;7(1):7386. doi: 10.1038/s41598-017-07782-9. Sci Rep. 2017. PMID: 28785097 Free PMC article.
-
Reducing the Antigen Prevalence Target Threshold for Stopping and Restarting Mass Drug Administration for Lymphatic Filariasis Elimination: A Model-Based Cost-effectiveness Simulation in Tanzania, India and Haiti.Clin Infect Dis. 2024 Apr 25;78(Suppl 2):S160-S168. doi: 10.1093/cid/ciae108. Clin Infect Dis. 2024. PMID: 38662697 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

