Safety of a Four-factor Prothrombin Complex Concentrate Versus Plasma for Vitamin K Antagonist Reversal: An Integrated Analysis of Two Phase IIIb Clinical Trials
- PMID: 26822172
- PMCID: PMC5071715
- DOI: 10.1111/acem.12911
Safety of a Four-factor Prothrombin Complex Concentrate Versus Plasma for Vitamin K Antagonist Reversal: An Integrated Analysis of Two Phase IIIb Clinical Trials
Abstract
Objectives: Clinicians often need to rapidly reverse vitamin K antagonists (VKAs) in the setting of major hemorrhage or urgent need for surgery. Little is known about the safety profile of the traditional reversal agent, plasma, or the newly approved agent, four-factor prothrombin complex concentrate (4F-PCC), in a randomized setting. This is an integrated analysis of safety data from two clinical trials that evaluated 4F-PCC versus plasma for the treatment of patients requiring rapid VKA reversal for acute major bleeding or prior to an urgent surgical/invasive procedure.
Methods: This descriptive analysis comprised adverse event (AE) data from two phase IIIb, randomized, controlled trials. The bleeding and surgical studies were performed across 36 and 33 sites, respectively, in nine countries, with the integrated analysis comprising 388 patients (4F-PCC, n = 191; plasma, n = 197) aged ≥ 18 years, who required VKA reversal due to major bleeding or prior to an urgent surgical/invasive procedure. Patients received either 4F-PCC, containing nonactivated factors II, VII, IX, and X and proteins C and S (Beriplex/Kcentra, CSL Behring) or plasma, both dosed according to baseline international normalized ratio and body weight. Patients were also to receive vitamin K1. AEs and serious AEs (SAEs) were assessed up to days 10 and 45, respectively.
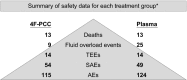
Results: The proportion of patients with AEs (4F-PCC, 115/191 [60.2%]; plasma, 124/197 [62.9%]) and SAEs (4F-PCC, 54/191 [28.3%]; plasma, 49/197 [24.9%]) was similar between groups. The proportion of patients with thromboembolic events was also similar between groups (4F-PCC, 14/191 [7.3%]; plasma, 14/197 [7.1%]). There were 13 (6.8%) deaths in the 4F-PCC group and 13 (6.6%) in the plasma group. Fluid overload events occurred in more patients in the plasma group than the 4F-PCC group (25 [12.7%] and 9 [4.7%], respectively).
Conclusions: These safety data represent the largest controlled assessment of a 4F-PCC to date. For patients requiring urgent VKA reversal, 4F-PCC had a safety profile similar to that of plasma (AEs, SAEs, thromboembolic events, and deaths), but was associated with fewer fluid overload events.
© 2016 by the Society for Academic Emergency Medicine.
Figures

References
-
- IMS data US National Prescription Audit. Danbury, CT: IMS Health, 2013.
-
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011;365:2002–12. - PubMed
-
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007;167:1414–9. - PubMed
-
- Levy JH, Tanaka KA, Dietrich W. Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology 2008;109:918–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

