Nucleophilic arylation with tetraarylphosphonium salts
- PMID: 26822205
- PMCID: PMC4740112
- DOI: 10.1038/ncomms10337
Nucleophilic arylation with tetraarylphosphonium salts
Abstract
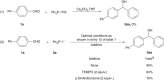
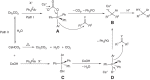
Organic phosphonium salts have served as important intermediates in synthetic chemistry. But the use of a substituent on the positive phosphorus as a nucleophile to construct C-C bond remains a significant challenge. Here we report an efficient transition-metal-free protocol for the direct nucleophilic arylation of carbonyls and imines with tetraarylphosphonium salts in the presence of caesium carbonate. The aryl nucleophile generated from phosphonium salt shows low basicity and good nucleophilicity, as evidenced by the successful conversion of enolizable aldehydes and ketones. The reaction is not particularly sensitive to water, shows wide substrate scope, and is compatible with a variety of functional groups including cyano and ester groups. Compared with the arylmetallic reagents that are usually moisture sensitive, the phosphonium salts are shelf-stable and can be easily handled.
Figures



References
-
- Modern Arylation Methods. ed. Ackermann Lutz Wiley-VCH, Verlag GmbH & Co. KGaA (2009) .
-
- Bruke A. J. & Marques C. S. Catalytic Arylation Methods - from the Academic Lab to Industrial Processes Wiley-VCH Verlag GmbH & Co. KGaA (2015) .
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

