SLC20A2 Deficiency in Mice Leads to Elevated Phosphate Levels in Cerbrospinal Fluid and Glymphatic Pathway-Associated Arteriolar Calcification, and Recapitulates Human Idiopathic Basal Ganglia Calcification
- PMID: 26822507
- PMCID: PMC4967033
- DOI: 10.1111/bpa.12362
SLC20A2 Deficiency in Mice Leads to Elevated Phosphate Levels in Cerbrospinal Fluid and Glymphatic Pathway-Associated Arteriolar Calcification, and Recapitulates Human Idiopathic Basal Ganglia Calcification
Abstract
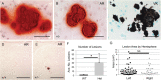
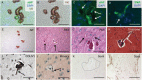
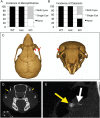
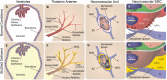
Idiopathic basal ganglia calcification is a brain calcification disorder that has been genetically linked to autosomal dominant mutations in the sodium-dependent phosphate co-transporter, SLC20A2. The mechanisms whereby deficiency of Slc20a2 leads to basal ganglion calcification are unknown. In the mouse brain, we found that Slc20a2 was expressed in tissues that produce and/or regulate cerebrospinal fluid, including choroid plexus, ependyma and arteriolar smooth muscle cells. Haploinsufficient Slc20a2 +/- mice developed age-dependent basal ganglia calcification that formed in glymphatic pathway-associated arterioles. Slc20a2 deficiency uncovered phosphate homeostasis dysregulation characterized by abnormally high cerebrospinal fluid phosphate levels and hydrocephalus, in addition to basal ganglia calcification. Slc20a2 siRNA knockdown in smooth muscle cells revealed increased susceptibility to high phosphate-induced calcification. These data suggested that loss of Slc20a2 led to dysregulated phosphate homeostasis and enhanced susceptibility of arteriolar smooth muscle cells to elevated phosphate-induced calcification. Together, dysregulated cerebrospinal fluid phosphate and enhanced smooth muscle cell susceptibility may predispose to glymphatic pathway-associated arteriolar calcification.
Keywords: Slc20a2; cerebral vascular calcification; cerebrospinal fluid; glymphatic spaces; idiopathic basal ganglia calcification; phosphate.
© 2016 International Society of Neuropathology.
Figures




Comment in
-
New Studies on Knockout Mouse for the SLC20A2 Gene Show Much More Than Brain Calcifications.J Mol Neurosci. 2016 Aug;59(4):565-6. doi: 10.1007/s12031-016-0778-8. Epub 2016 Jul 6. J Mol Neurosci. 2016. PMID: 27380911 No abstract available.
References
-
- Chen WJ et al (2013) Novel SLC20A2 mutations identified in southern Chinese patients with idiopathic basal ganglia calcification. Gene 529:159–162. - PubMed
-
- Cheng SL, Shao JS, Charlton‐Kachigian N, Loewy AP, Towler DA (2003) MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem 278:45969–45977. - PubMed
-
- Demoulin JB et al (2004) Platelet‐derived growth factor stimulates membrane lipid synthesis through activation of phosphatidylinositol 3‐kinase and sterol regulatory element‐binding proteins. J Biol Chem 279:35392–35402. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

