BPA-toxicity via superoxide anion overload and a deficit in β-catenin signaling in human bone mesenchymal stem cells
- PMID: 26822619
- PMCID: PMC5217073
- DOI: 10.1002/tox.22239
BPA-toxicity via superoxide anion overload and a deficit in β-catenin signaling in human bone mesenchymal stem cells
Abstract
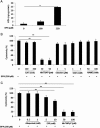
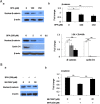
Bisphenol A (BPA), used in the manufacture of products based on polycarbonate plastics and epoxy resins, is well known as an endocrine-disrupting monomer. In the current study, BPA increased cytotoxicity in hBMSCs in a dose- and time-dependent manner, concomitantly with increased lipid peroxidation. Increased cell death in BPA-treated cells was markedly blocked by pretreatment with the superoxide dismutase mimetic MnTBAP and MnTMPyP, but not by catalase, glutathione, the glutathione peroxidase mimetic ebselen, the NOS inhibitor NAME, or the xanthine oxidase inhibitor allopurinol. Furthermore, the decline in nuclear β-catenin and cyclin D1 levels in hBMSCs exposed to BPA was reversed by MnTBAP treatment. Finally, treatment of hBMSCs with the GSK3β inhibitor LiCl2 increased nuclear β-catenin levels and significantly attenuated cytotoxicity compared with BPA treatment. Our current results in hBMSCs exposed to BPA suggest that BPA causes a disturbance in β-catenin signaling via a superoxide anion overload. © 2016 The Authors Environmental Toxicology Published by Wiley Periodicals, Inc. Environ Toxicol 32: 344-352, 2017.
Keywords: GSK3β; MnTBAP; bisphenol A; human bone mesenchymal stem cells; superoxide dismutase; β-catenin.
© 2016 The Authors Environmental Toxicology Published by Wiley Periodicals, Inc.
Figures


Similar articles
-
Effects of bisphenol A on antioxidant system in soybean seedling roots.Environ Toxicol Chem. 2015 May;34(5):1127-33. doi: 10.1002/etc.2904. Epub 2015 Apr 7. Environ Toxicol Chem. 2015. PMID: 25651304
-
Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats.Environ Toxicol. 2016 Dec;31(12):1922-1934. doi: 10.1002/tox.22193. Epub 2015 Oct 9. Environ Toxicol. 2016. PMID: 26450347
-
Bisphenol A Induces Oxidative Stress in Bone Marrow Cells, Lymphocytes, and Reproductive Organs of Holtzman Rats.Int J Toxicol. 2017 Mar/Apr;36(2):142-152. doi: 10.1177/1091581817691224. Epub 2017 Mar 21. Int J Toxicol. 2017. PMID: 28403740
-
Effects of Bisphenol A on ion channels: Experimental evidence and molecular mechanisms.Steroids. 2016 Jul;111:12-20. doi: 10.1016/j.steroids.2016.02.020. Epub 2016 Feb 27. Steroids. 2016. PMID: 26930576 Review.
-
Neurological Effects of Bisphenol A and its Analogues.Int J Med Sci. 2015 Oct 30;12(12):926-36. doi: 10.7150/ijms.13267. eCollection 2015. Int J Med Sci. 2015. PMID: 26664253 Free PMC article. Review.
Cited by
-
Molecular and cellular mechanisms linking air pollution and bone damage.Environ Res. 2020 Jun;185:109465. doi: 10.1016/j.envres.2020.109465. Epub 2020 Apr 6. Environ Res. 2020. PMID: 32305664 Free PMC article. Review.
-
Bisphenol-A Abrogates Proliferation and Differentiation of C2C12 Mouse Myoblasts via Downregulation of Phospho-P65 NF-κB Signaling Pathway.J Toxicol. 2024 Feb 28;2024:3840950. doi: 10.1155/2024/3840950. eCollection 2024. J Toxicol. 2024. PMID: 38449520 Free PMC article.
-
Induction of oxidative stress by bisphenol A and its pleiotropic effects.Environ Mol Mutagen. 2017 Mar;58(2):60-71. doi: 10.1002/em.22072. Epub 2017 Feb 9. Environ Mol Mutagen. 2017. PMID: 28181297 Free PMC article. Review.
-
Effects of environmental stressors on stem cells.World J Stem Cells. 2019 Sep 26;11(9):565-577. doi: 10.4252/wjsc.v11.i9.565. World J Stem Cells. 2019. PMID: 31616535 Free PMC article. Review.
-
Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions.Pharmaceutics. 2023 Mar 10;15(3):908. doi: 10.3390/pharmaceutics15030908. Pharmaceutics. 2023. PMID: 36986769 Free PMC article. Review.
References
-
- An WG, Kanekal M, Simon MC, Maltepe E, Blagoslonny MV, Neckers LM. 1998. Stabilization of wild‐type p53 by hypoxia‐inducible factor 1alpha. Nature 392:405–408. - PubMed
-
- Aydogan M, Korkmaz A, Barlas N, Kolankaya D. 2008. The effect of vitamin C on bisphenol A, nonylphenol and octylphenol induced brain damages of male rats. Toxicology 249:35–39. - PubMed
-
- Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW. 2007. Generation of mulitipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J Biol Chem 282:31703–31712. - PubMed
-
- Barnham KJ, Masters CL, Bush AI. 2004. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214. - PubMed
-
- Bedard K, Krause KH. 2007. The NOX family of ROS‐generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev 87:245–313. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

