Six3 regulates optic nerve development via multiple mechanisms
- PMID: 26822689
- PMCID: PMC4731751
- DOI: 10.1038/srep20267
Six3 regulates optic nerve development via multiple mechanisms
Abstract
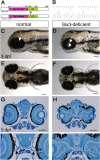
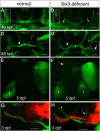
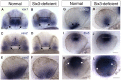
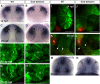
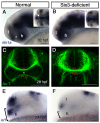
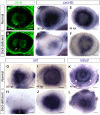
Malformations of the optic nerve lead to reduced vision or even blindness. During optic nerve development, retinal ganglion cell (RGC) axons navigate across the retina, exit the eye to the optic stalk (OS), and cross the diencephalon midline at the optic chiasm en route to their brain targets. Many signalling molecules have been implicated in guiding various steps of optic nerve pathfinding, however much less is known about transcription factors regulating this process. Here we show that in zebrafish, reduced function of transcription factor Six3 results in optic nerve hypoplasia and a wide repertoire of RGC axon pathfinding errors. These abnormalities are caused by multiple mechanisms, including abnormal eye and OS patterning and morphogenesis, abnormal expression of signalling molecules both in RGCs and in their environment and anatomical deficiency in the diencephalic preoptic area, where the optic chiasm normally forms. Our findings reveal new roles for Six3 in eye development and are consistent with known phenotypes of reduced SIX3 function in humans. Hence, the new zebrafish model for Six3 loss of function furthers our understanding of the mechanisms governing optic nerve development and Six3-mediated eye and forebrain malformations.
Figures

Similar articles
-
Expression of a Barhl1a reporter in subsets of retinal ganglion cells and commissural neurons of the developing zebrafish brain.Sci Rep. 2020 Jun 1;10(1):8814. doi: 10.1038/s41598-020-65435-w. Sci Rep. 2020. PMID: 32483163 Free PMC article.
-
Expression of two zebrafish homologues of the murine Six3 gene demarcates the initial eye primordia.Mech Dev. 1998 Apr;73(1):45-57. doi: 10.1016/s0925-4773(98)00028-8. Mech Dev. 1998. PMID: 9545529
-
Overexpression of the forebrain-specific homeobox gene six3 induces rostral forebrain enlargement in zebrafish.Development. 1998 Aug;125(15):2973-82. doi: 10.1242/dev.125.15.2973. Development. 1998. PMID: 9655819
-
Signaling - transcription interactions in mouse retinal ganglion cells early axon pathfinding -a literature review.Front Ophthalmol (Lausanne). 2023 May 17;3:1180142. doi: 10.3389/fopht.2023.1180142. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983012 Free PMC article. Review.
-
Glia, neurons, and axon pathfinding during optic chiasm development.Curr Opin Neurobiol. 1997 Oct;7(5):647-53. doi: 10.1016/s0959-4388(97)80084-0. Curr Opin Neurobiol. 1997. PMID: 9384544 Review.
Cited by
-
Zebrafish model of RERE syndrome recapitulates key ophthalmic defects that are rescued by small molecule inhibitor of shh signaling.Dev Dyn. 2023 Apr;252(4):495-509. doi: 10.1002/dvdy.561. Epub 2023 Jan 10. Dev Dyn. 2023. PMID: 36576487 Free PMC article.
-
SSBP1 mutations cause mtDNA depletion underlying a complex optic atrophy disorder.J Clin Invest. 2020 Jan 2;130(1):108-125. doi: 10.1172/JCI128514. J Clin Invest. 2020. PMID: 31550240 Free PMC article.
-
Magnetic nanoparticles: a strategy to target the choroidal layer in the posterior segment of the eye.Sci Rep. 2017 Mar 3;7:43092. doi: 10.1038/srep43092. Sci Rep. 2017. PMID: 28256525 Free PMC article.
-
VEP analysis methods in children with optic nerve hypoplasia: relationship to visual acuity and optic disc diameter.Doc Ophthalmol. 2016 Dec;133(3):159-169. doi: 10.1007/s10633-016-9566-6. Epub 2016 Nov 23. Doc Ophthalmol. 2016. PMID: 27882486
-
Six3 in a small population of progenitors at E8.5 is required for neuroretinal specification via regulating cell signaling and survival in mice.Dev Biol. 2017 Aug 1;428(1):164-175. doi: 10.1016/j.ydbio.2017.05.026. Epub 2017 Jun 1. Dev Biol. 2017. PMID: 28579317 Free PMC article.
References
-
- Oster S. F., Deiner M., Birgbauer E. & Sretavan D. W. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol 15, 125–136 (2004). - PubMed
-
- Petros T. J., Rebsam A. & Mason C. A. Retinal axon growth at the optic chiasm: to cross or not to cross. Annu Rev Neurosci 31, 295–315 (2008). - PubMed
-
- Baier H. et al.. Genetic dissection of the retinotectal projection. Development 123, 415–425 (1996). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

