Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study
- PMID: 26822714
- PMCID: PMC4731911
- DOI: 10.1186/s12937-016-0130-8
Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study
Abstract
Background: Undenatured type II collagen (UC-II) is a nutritional supplement derived from chicken sternum cartilage. The purpose of this study was to evaluate the efficacy and tolerability of UC-II for knee osteoarthritis (OA) pain and associated symptoms compared to placebo and to glucosamine hydrochloride plus chondroitin sulfate (GC).
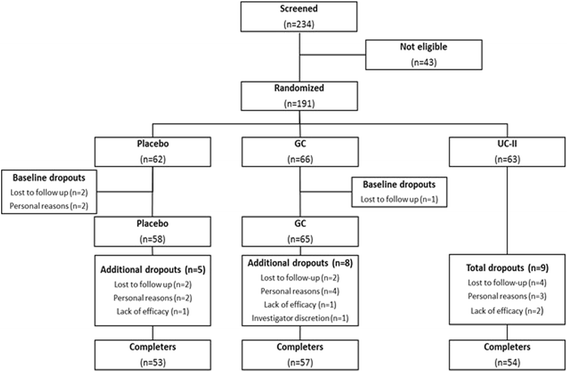
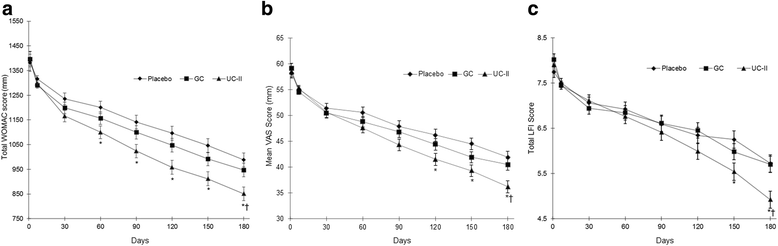
Methods: One hundred ninety one volunteers were randomized into three groups receiving a daily dose of UC-II (40 mg), GC (1500 mg G & 1200 mg C), or placebo for a 180-day period. The primary endpoint was the change in total Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) from baseline through day 180 for the UC-II group versus placebo and GC. Secondary endpoints included the Lequesne Functional Index (LFI), the Visual Analog Scale (VAS) for pain and the WOMAC subscales. Modified intent-to-treat analysis were performed for all endpoints using analysis of covariance and mixed model repeated measures, while incremental area under the curve was calculated by the intent-to-treat method.
Results: At day 180, the UC-II group demonstrated a significant reduction in overall WOMAC score compared to placebo (p = 0.002) and GC (p = 0.04). Supplementation with UC-II also resulted in significant changes for all three WOMAC subscales: pain (p = 0.0003 vs. placebo; p = 0.016 vs. GC); stiffness (p = 0.004 vs. placebo; p = 0.044 vs. GC); physical function (p = 0.007 vs. placebo). Safety outcomes did not differ among the groups.
Conclusion: UC-II improved knee joint symptoms in knee OA subjects and was well-tolerated. Additional studies that elucidate the mechanism for this supplement's actions are warranted.
Trial registration: CTRI/2013/05/003663 ; CTRI/2013/02/003348 .
Figures
Similar articles
-
A 2-week, multicenter, randomized, double-blind, placebo-controlled, dose-ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee.Clin Ther. 2006 Mar;28(3):352-64. doi: 10.1016/j.clinthera.2006.03.008. Clin Ther. 2006. PMID: 16750450 Clinical Trial.
-
Combined Treatment With Chondroitin Sulfate and Glucosamine Sulfate Shows No Superiority Over Placebo for Reduction of Joint Pain and Functional Impairment in Patients With Knee Osteoarthritis: A Six-Month Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial.Arthritis Rheumatol. 2017 Jan;69(1):77-85. doi: 10.1002/art.39819. Arthritis Rheumatol. 2017. PMID: 27477804 Clinical Trial.
-
A 12-week randomized, double-blind, placebo-controlled multicenter study of choline-stabilized orthosilicic acid in patients with symptomatic knee osteoarthritis.BMC Musculoskelet Disord. 2017 Jan 5;18(1):2. doi: 10.1186/s12891-016-1370-7. BMC Musculoskelet Disord. 2017. PMID: 28056936 Free PMC article. Clinical Trial.
-
Efficacy of undenatured collagen in knee osteoarthritis: review of the literature with limited meta-analysis.Am J Transl Res. 2023 Sep 15;15(9):5545-5555. eCollection 2023. Am J Transl Res. 2023. PMID: 37854210 Free PMC article. Review.
-
Undenatured type II collagen for knee osteoarthritis.Ann Med. 2025 Dec;57(1):2493306. doi: 10.1080/07853890.2025.2493306. Epub 2025 Apr 20. Ann Med. 2025. PMID: 40253594 Free PMC article. Review.
Cited by
-
Undenatured Type II Collagen Ameliorates Inflammatory Responses and Articular Cartilage Damage in the Rat Model of Osteoarthritis.Front Vet Sci. 2021 Mar 4;8:617789. doi: 10.3389/fvets.2021.617789. eCollection 2021. Front Vet Sci. 2021. PMID: 33748207 Free PMC article.
-
Physicochemical properties of acid- and pepsin-soluble collagens from the cartilage of Siberian sturgeon.Environ Sci Pollut Res Int. 2018 Nov;25(31):31427-31438. doi: 10.1007/s11356-018-3147-z. Epub 2018 Sep 8. Environ Sci Pollut Res Int. 2018. PMID: 30196466
-
Healthcare and Scientific Treatment of Knee Osteoarthritis.J Healthc Eng. 2022 Jan 28;2022:5919686. doi: 10.1155/2022/5919686. eCollection 2022. J Healthc Eng. 2022. Retraction in: J Healthc Eng. 2023 Dec 6;2023:9815921. doi: 10.1155/2023/9815921. PMID: 35126931 Free PMC article. Retracted. Review.
-
Collagen supplementation in skin and orthopedic diseases: A review of the literature.Heliyon. 2023 Mar 28;9(4):e14961. doi: 10.1016/j.heliyon.2023.e14961. eCollection 2023 Apr. Heliyon. 2023. PMID: 37064452 Free PMC article. Review.
-
Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids.Int J Mol Sci. 2021 Jan 8;22(2):580. doi: 10.3390/ijms22020580. Int J Mol Sci. 2021. PMID: 33430111 Free PMC article.
References
-
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–74. doi: 10.1002/acr.21596. - DOI - PubMed
-
- National Institute of Health and Care Excellence; NICE clinical guideline 177. https://www.nice.org.uk/guidance/cg177. accessed October 19, 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

