Accuracy of clinical criteria and an immunochromatographic strip test for dengue diagnosis in a DENV-4 epidemic
- PMID: 26822788
- PMCID: PMC4731992
- DOI: 10.1186/s12879-016-1368-7
Accuracy of clinical criteria and an immunochromatographic strip test for dengue diagnosis in a DENV-4 epidemic
Abstract
Background: Early diagnosis of dengue infection is important for decision-making and timely implementation of therapeutic measures. Although rapid NS1 assays have been used for dengue diagnosis since 2008, their performance in DENV-4 cases has not yet been fully assessed.
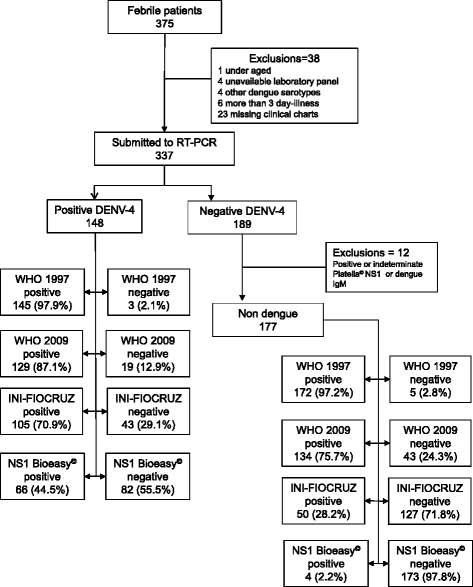
Methods: We evaluated the accuracy of NS1 Bioeasy™ immunochromatographic strip test and of three clinical criteria for dengue diagnosis. Patients presenting at an emergency care center within 72 h of an acute febrile illness during the 2013 DENV-4 epidemic in Rio de Janeiro were consecutively enrolled for clinical and laboratory evaluation. We classified patients as suspected dengue or not according to three clinical criteria: WHO 2009, WHO 1997, and INI-FIOCRUZ. Dengue diagnosis was defined by RNA detection using RT-PCR and the negative cases were negative for all dengue serotypes and also Platelia™ NS1 ELISA. We obtained accuracy indices for NS1 Bioeasy™ alone and in combination with the clinical criteria.
Results: RT-PCR for DENV-4 was positive in 148 out of 325 patients. Positive likelihood ratio, sensitivity, and specificity of NS1 Bioeasy™ with WHO 2009, WHO 1997, and INI-FIOCRUZ criteria were 22.6 (95% CI 7.2-70.6), 40.6% (95% CI 32.3-49.3), and 98.2% (95% CI 94.9-99.6); 18.3 (95% CI 6.8-49.2), 44.2 (95% CI 35.8-52.9), 97.6 (95% CI 94.0-99.3); 26.2 (95% CI 6.5-106.5), 29.7 (95% CI 22.4-37.8), 98.9 (95% CI 96.0-99.9), respectively. WHO 1997 clinical criteria presented high sensitivity to rule out disease, but extremely low specificity. INI-FIOCRUZ had moderate sensitivity and specificity, and could target a group to a more specific test.
Conclusions: Although the large rates of false negative results using NS1 Bioeasy™ rapid test advise against its use for triaging (rule out) purposes in DENV-4 epidemics, it could be used as a confirmatory tool in a bedside algorithm.
Figures
Similar articles
-
Precisão e confiabilidade de um teste imuno-cromatográfico rápido NS1 para diagnóstico DENV-1 no ponto de atendimento e no laboratório.BMC Infect Dis. 2017 Aug 29;17(1):594. doi: 10.1186/s12879-017-2679-z. BMC Infect Dis. 2017. PMID: 28851293 Free PMC article.
-
Diagnostic parameters and reliability of four rapid immunochromatographic tests for dengue 4.Braz J Infect Dis. 2020 Jan-Feb;24(1):58-64. doi: 10.1016/j.bjid.2019.12.004. Epub 2020 Jan 16. Braz J Infect Dis. 2020. PMID: 31954721 Free PMC article.
-
Utility of dengue NS1 antigen rapid diagnostic test for use in difficult to reach areas and its comparison with dengue NS1 ELISA and qRT-PCR.J Med Virol. 2017 Jul;89(7):1146-1150. doi: 10.1002/jmv.24764. Epub 2017 Feb 27. J Med Virol. 2017. PMID: 28042883
-
False-Positive Nonstructural Protein 1 Antigen in a Patient with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Case Report with Literature Review.Am J Case Rep. 2021 Apr 9;22:e928865. doi: 10.12659/AJCR.928865. Am J Case Rep. 2021. PMID: 33835996 Free PMC article. Review.
-
Rapid immunochromatographic tests for the diagnosis of dengue: a systematic review and meta-analysis.Cad Saude Publica. 2020 Jun 8;36(6):e00225618. doi: 10.1590/0102-311X00225618. eCollection 2020. Cad Saude Publica. 2020. PMID: 32520127
Cited by
-
Effectiveness of a diagnostic algorithm for dengue based on an artificial neural network.Digit Health. 2024 Mar 5;10:20552076241237691. doi: 10.1177/20552076241237691. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 38449678 Free PMC article.
-
Lessons from South Korea Regarding the Early Stage of the COVID-19 Outbreak.Healthcare (Basel). 2020 Jul 24;8(3):229. doi: 10.3390/healthcare8030229. Healthcare (Basel). 2020. PMID: 32722174 Free PMC article.
-
Towards Multiplex Molecular Diagnosis-A Review of Microfluidic Genomics Technologies.Micromachines (Basel). 2017 Aug 30;8(9):266. doi: 10.3390/mi8090266. Micromachines (Basel). 2017. PMID: 30400456 Free PMC article. Review.
-
Development and validation of a clinical rule for the diagnosis of chikungunya fever in a dengue-endemic area.PLoS One. 2023 Jan 6;18(1):e0279970. doi: 10.1371/journal.pone.0279970. eCollection 2023. PLoS One. 2023. PMID: 36608030 Free PMC article.
-
Validation and reliability of the rapid diagnostic test 'SD Bioeasy Dengue Duo' for dengue diagnosis in Brazil: a phase III study.Mem Inst Oswaldo Cruz. 2018 Jun 25;113(8):e170433. doi: 10.1590/0074-02760170433. Mem Inst Oswaldo Cruz. 2018. PMID: 29947711 Free PMC article. Clinical Trial.
References
-
- World Health Organization . Dengue: diagnosis, treatment, prevention and control. Geneva: WHO; 2009. - PubMed
-
- Dietz VJ, Gubler DJ, Rigau-Perez JG, Pinheiro F, Schatzmayr HG, Bailey R, et al. Epidemic dengue 1 in Brazil, 1986: evaluation of a clinically based dengue surveillance system. Am J Epidemiol. 1990;131(4):693–701. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical