Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine
- PMID: 26822914
- PMCID: PMC4867328
- DOI: 10.1152/ajpgi.00314.2015
Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine
Abstract
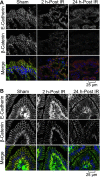
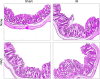
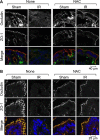
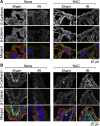
The goals of this study were to evaluate the effects of ionizing radiation on apical junctions in colonic epithelium and mucosal barrier function in mice in vivo. Adult mice were subjected to total body irradiation (4 Gy) with or without N-acetyl-l-cysteine (NAC) feeding for 5 days before irradiation. At 2-24 h postirradiation, the integrity of colonic epithelial tight junctions (TJ), adherens junctions (AJ), and the actin cytoskeleton was assessed by immunofluorescence microscopy and immunoblot analysis of detergent-insoluble fractions for TJ and AJ proteins. The barrier function was evaluated by measuring vascular-to-luminal flux of fluorescein isothiocyanate (FITC)-inulin in vivo and luminal-to-mucosal flux in vitro. Oxidative stress was evaluated by measuring protein thiol oxidation. Confocal microscopy showed that radiation caused redistribution of occludin, zona occludens-1, claudin-3, E-cadherin, and β-catenin, as well as the actin cytoskeleton as early as 2 h postirradiation, and this effect was sustained for at least 24 h. Feeding NAC before irradiation blocked radiation-induced disruption of TJ, AJ, and the actin cytoskeleton. Radiation increased mucosal permeability to inulin in colon, which was blocked by NAC feeding. The level of reduced-protein thiols in colon was depleted by radiation with a concomitant increase in the level of oxidized-protein thiol. NAC feeding blocked the radiation-induced protein thiol oxidation. These data demonstrate that radiation rapidly disrupts TJ, AJ, and the actin cytoskeleton by an oxidative stress-dependent mechanism that can be prevented by NAC feeding.
Keywords: actin; adherens junction; barrier function; cytoskeleton; intestine; occludin; oxidative stress; protein thiol; zona occludens-1.
Copyright © 2016 the American Physiological Society.
Figures







References
-
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6: 593–597, 1989. - PubMed
-
- Baier JE, Neumann HA, Moeller T, Kissler M, Borchardt D, Ricken D. Radiation protection through cytokine release by N-acetylcysteine. Strahlenther Onkol 172: 91–98, 1996. - PubMed
-
- Cameron S, Schwartz A, Sultan S, Schaefer IM, Hermann R, Rave-Frank M, Hess CF, Christiansen H, Ramadori G. Radiation-induced damage in different segments of the rat intestine after external beam irradiation of the liver. Exp Mol Pathol 92: 243–258, 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

