Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry
- PMID: 26822918
- PMCID: PMC4996118
- DOI: 10.1093/eurheartj/ehv730
Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry
Abstract
Aims: Left atrial appendage closure is a non-pharmacological alternative for stroke prevention in high-risk patients with non-valvular atrial fibrillation. The objective of the multicentre EWOLUTION registry was to obtain clinical data on procedural success and complications, and long-term patient outcomes, including bleeding and incidence of stroke/transient ischaemic attack (TIA). Here, we report on the peri-procedural outcomes of up to 30 days.
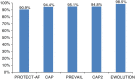
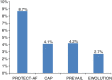
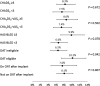
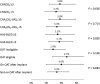
Methods and results: Baseline/implant data are available for 1021 subjects. Subjects in the study were at high risk of stroke (average CHADS2 score: 2.8 ± 1.3, CHA2DS2-VASc: 4.5 ± 1.6) and moderate-to-high risk of bleeding (average HAS-BLED score: 2.3 ± 1.2). Almost half of the subjects (45.4%) had a history of TIA, ischaemic stroke, or haemorrhagic stroke; 62% of patients were deemed unsuitable for novel oral anticoagulant by their physician. The device was successfully deployed in 98.5% of patients with no flow or minimal residual flow achieved in 99.3% of implanted patients. Twenty-eight subjects experienced 31 serious adverse events (SAEs) within 1 day of the procedure. The overall 30-day mortality rate was 0.7%. The most common SAE occurring within 30 days of the procedure was major bleeding requiring transfusion. Incidence of SAEs within 30 days was significantly lower for subjects deemed to be ineligible for oral anticoagulation therapy (OAT) compared with those eligible for OAT (6.5 vs. 10.2%, P = 0.042).
Conclusion: Left atrial appendage closure with the WATCHMAN device has a high success rate in complete LAAC with low peri-procedural risk, even in a population with a higher risk of stroke and bleeding, and multiple co-morbidities. Improvement in implantation techniques has led to a reduction of peri-procedural complications previously limiting the net clinical benefit of the procedure.
Keywords: Atrial fibrillation; Left atrial appendage; Stroke.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


Comment in
-
The evolution of left atrial appendage occlusion: EWOLUTION and the WATCHMAN in practice.Eur Heart J. 2016 Aug;37(31):2475-7. doi: 10.1093/eurheartj/ehw041. Epub 2016 Apr 28. Eur Heart J. 2016. PMID: 27125946 No abstract available.
References
-
- Blackshear J , Odell J . Appendage obliteration to reduce stroke in cardiac surgical patients with AF . Ann Thorac Surg 1996. ; 61 : 755 – 759 . - PubMed
-
- Holmes DR , Reddy VY , Doshi SK , Sievert H , Buchbinder M , Neuzil P , Huber K , Halperin J , Holmes D , on behalf of the PROTECT AF Investigators . Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial . Lancet 2009. ; 374 : 534 – 542 . - PubMed
-
- Holmes DR Jr , Kar S , Price MJ , Whisenant B , Sievert H , Doshi SK , Huber K , Reddy VY . Prospective randomized evaluation of the WATCHMAN left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial . J Am Coll Cardiol 2014. ; 64 : 1 – 12 . - PubMed
-
- Reddy VY , Sievert H , Halperin J , Doshi SK , Buchbinder M , Neuzil P , Huber K , Whisenant B , Kar S , Swarup V , Gordon N , Holmes D , for the PROTECT AF Steering Committee and Investigators . Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial . JAMA 2014. ; 312 : 1988 – 1998 . - PubMed
-
- Camm AJ , Lip GY , De Caterina S , Savelieva I , Atar D , Hohnloser SH , Hindricks G , Kirchhof P , ESC Committee for Practice Guidelines (CPG) . 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation: developed with the special contribution of the European Heart Rhythm Association . Eur Heart J 2012. ; 33 : 2719 – 2747 . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

