In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality
- PMID: 26823067
- PMCID: PMC5227679
- DOI: 10.4103/1008-682X.170863
In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality
Abstract
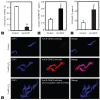
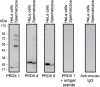
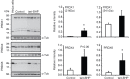
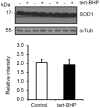
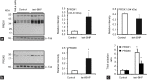
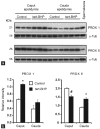
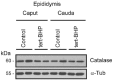
Oxidative stress, the imbalance between the production of reactive oxygen species (ROS) and antioxidant activity is a major culprit of male infertility. Peroxiredoxins (PRDXs) are major antioxidant enzymes of mammalian spermatozoa and are thiol oxidized and inactivated by ROS in a dose-dependent manner. Their deficiency and/or inactivation have been associated with men infertility. The aim of this study was to elucidate the impact of oxidative stress, generated by the in vivo tert-butyl hydroperoxide (tert-BHP) treatment on rat epididymal spermatozoa during their maturation process. Adult Sprague-Dawley males were treated with 300 μmoles tert-BHP/kg or saline (control) per day intraperitoneal for 15 days. Lipid peroxidation (2-thibarbituric acid reactive substances assay), total amount and thiol oxidation of PRDXs along with the total amount of superoxide dismutase (SOD), motility and DNA oxidation (8-hydroxy-deoxyguanosine) were determined in epididymal spermatozoa. Total amount of PRDXs and catalase and thiol oxidation of PRDXs were determined in caput and cauda epididymis. While animals were not affected by treatment, their epididymal spermatozoa have decreased motility, increased levels of DNA oxidation and lipid peroxidation along with increased PRDXs (and not SOD) amounts. Moreover, sperm PRDXs were highly thiol oxidized. There was a differential regulation in the expression of PRDX1 and PRDX6 in the epididymis that suggests a segment-specific role for PRDXs. In conclusion, PRDXs are increased in epididymal spermatozoa in an attempt to fight against the oxidative stress generated by tert-BHP in the epididymis. These findings highlight the role of PRDXs in the protection of sperm function and DNA integrity during epididymal maturation.
Figures
Similar articles
-
Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men.J Androl. 2012 Nov-Dec;33(6):1342-51. doi: 10.2164/jandrol.111.016162. Epub 2012 Apr 5. J Androl. 2012. PMID: 22492841
-
Absence of Peroxiredoxin 6 Amplifies the Effect of Oxidant Stress on Mobility and SCSA/CMA3 Defined Chromatin Quality and Impairs Fertilizing Ability of Mouse Spermatozoa.Biol Reprod. 2016 Mar;94(3):68. doi: 10.1095/biolreprod.115.137646. Epub 2016 Jan 20. Biol Reprod. 2016. PMID: 26792942 Free PMC article.
-
Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa.Hum Reprod. 2018 Aug 1;33(8):1394-1407. doi: 10.1093/humrep/dey221. Hum Reprod. 2018. PMID: 29912414 Free PMC article.
-
OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: The protection of mammalian spermatozoa against oxidative stress.Reproduction. 2022 Oct 26;164(6):F67-F78. doi: 10.1530/REP-22-0200. Print 2022 Dec 1. Reproduction. 2022. PMID: 37021966 Review.
-
Orchestrating the antioxidant defenses in the epididymis.Andrology. 2019 Sep;7(5):662-668. doi: 10.1111/andr.12630. Epub 2019 May 1. Andrology. 2019. PMID: 31044545 Review.
Cited by
-
Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization.Antioxidants (Basel). 2021 Jan 12;10(1):98. doi: 10.3390/antiox10010098. Antioxidants (Basel). 2021. PMID: 33445610 Free PMC article. Review.
-
Effects of Date Palm Pollen Supplementations on The Expression of PRDX1 and PRDX6 Genes in Infertile Men: A Controlled Clinical Trial.Int J Fertil Steril. 2023 Apr 1;17(3):201-207. doi: 10.22074/ijfs.2022.549291.1264. Int J Fertil Steril. 2023. PMID: 37183847 Free PMC article.
-
Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa.Antioxidants (Basel). 2020 Feb 19;9(2):170. doi: 10.3390/antiox9020170. Antioxidants (Basel). 2020. PMID: 32093059 Free PMC article.
-
Effects of administration of co-trimoxazole and folic acid on sperm quality and histological changes of testes in male rats.Int J Reprod Biomed. 2017 Oct;15(10):625-634. Int J Reprod Biomed. 2017. PMID: 29387828 Free PMC article.
-
Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility.Antioxidants (Basel). 2020 Feb 4;9(2):134. doi: 10.3390/antiox9020134. Antioxidants (Basel). 2020. PMID: 32033035 Free PMC article. Review.
References
-
- World Health Organization. Towards more objectivity in diagnosis and management of male fertility. Int J Androl. 1997;(Suppl, issue 7):1–53.
-
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 1992;57:409–16. - PubMed
-
- Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update. 2008;14:243–58. - PubMed
-
- Halliwell B, Gutteridge J, editors. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. Antioxidant defences: Endogenous and diet derived; pp. 79–186.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

