A Randomized, Controlled Trial of the Impact of Alternative Dosing Schedules on the Immune Response to Human Rotavirus Vaccine in Rural Ghanaian Infants
- PMID: 26823335
- PMCID: PMC4857471
- DOI: 10.1093/infdis/jiw023
A Randomized, Controlled Trial of the Impact of Alternative Dosing Schedules on the Immune Response to Human Rotavirus Vaccine in Rural Ghanaian Infants
Erratum in
-
Armah et al (J Infect Dis 2016; 213:1678-85).J Infect Dis. 2016 Oct 1;214(7):1127. doi: 10.1093/infdis/jiw265. Epub 2016 Aug 2. J Infect Dis. 2016. PMID: 27485358 Free PMC article. No abstract available.
Abstract
Background: The recommended schedule for receipt of 2-dose human rotavirus vaccine (HRV) coincides with receipt of the first and second doses of diphtheria, pertussis, and tetanus vaccine (ie, 6 and 10 weeks of age, respectively). Alternative schedules and additional doses of HRV have been proposed and may improve vaccine performance in low-income countries.
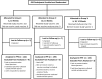
Methods: In this randomized trial in rural Ghana, HRV was administered at ages 6 and 10 weeks (group 1), 10 and 14 weeks (group 2), or 6, 10, and 14 weeks (group 3). We compared serum antirotavirus immunoglobulin A (IgA) seroconversion (≥20 U/mL) and geometric mean concentrations (GMCs) between group 1 and groups 2 and 3.
Results: Ninety-three percent of participants (424 of 456) completed the study per protocol. In groups 1, 2, and 3, the IgA seroconversion frequencies among participants with IgA levels of <20 U/mL at baseline were 28.9%, 37.4%, and 43.4%, respectively (group 1 vs group 3, P = .014; group 1 vs group 2, P = .163). Postvaccination IgA GMCs were 22.1 U/mL, 26.5 U/mL, and 32.6 U/mL in groups 1, 2, and 3, respectively (group 1 vs group 3, P = .038; group 1 vs group 2, P = .304).
Conclusions: A third dose of HRV resulted in increased seroconversion frequencies and GMCs, compared with 2 doses administered at 6 and 10 weeks of age. Since there is no correlate of protection, a postmarketing effectiveness study is required to determine whether the improvement in immune response translates into a public health benefit in low-income countries.
Clinical trials registration: NCT015751.
Trial registration: ClinicalTrials.gov NCT01575197.
Keywords: developing countries; immunization schedules; immunogenicity; infant; randomized controlled trial; rotavirus; rotavirus vaccines; vaccines.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Can Changes to Scheduling Enhance the Performance of Rotavirus Vaccines in Low-Income Countries?J Infect Dis. 2016 Jun 1;213(11):1673-5. doi: 10.1093/infdis/jiw026. Epub 2016 Jan 27. J Infect Dis. 2016. PMID: 26823336 No abstract available.
References
-
- World Health Organization. Rotavirus vaccines WHO position paper. Wkly Epidemiol Rec 2007; 82:285–96. - PubMed
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136–41. - PubMed
-
- Armah GE, Breiman RF, Tapia MD et al. . Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine 2012; 30(suppl 1):A86–93. - PubMed
-
- Binka FN, Anto FK, Oduro AR et al. . Incidence and risk factors of paediatric rotavirus diarrhoea in northern Ghana. Trop Med Int Health 2003; 8:840–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous