Noninterference of Rotavirus Vaccine With Measles-Rubella Vaccine at 9 Months of Age and Improvements in Antirotavirus Immunity: A Randomized Trial
- PMID: 26823338
- PMCID: PMC4857472
- DOI: 10.1093/infdis/jiw024
Noninterference of Rotavirus Vaccine With Measles-Rubella Vaccine at 9 Months of Age and Improvements in Antirotavirus Immunity: A Randomized Trial
Abstract
Background: The burden of rotavirus morbidity and mortality is high in children aged <5 years in developing countries, and evaluations indicate waning protection from rotavirus immunization in the second year. An additional dose of rotavirus vaccine may enhance the immune response and lengthen the period of protection against disease, but coadministration of this dose should not interfere with immune responses to concurrently given vaccines.
Methods: A total of 480 9-month-old participants from Matlab, Bangladesh, were enrolled in a study with a primary objective to establish noninferiority of concomitant administration of measles-rubella vaccine (MR) and a third dose of human rotavirus vaccine (HRV; MR + HRV), compared with MR given alone. Secondary objectives included noninferiority of rubella antibody seroconversion and evaluating rotavirus IgA/IgG seroresponses in MR + HRV recipients.
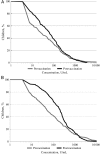
Results: Two months after vaccination, 75.3% and 74.3% of MR + HRV and MR recipients, respectively, had seroprotective levels of measles virus antibodies; 100.0% and 99.6%, respectively, showed anti-rubella virus immunoglobulin G (IgG) seroprotection. In the MR + HRV group, antirotavirus immunoglobulin A and IgG seropositivity frequencies before vaccination (52.7% and 66.3%, respectively) increased to 69.6% and 88.3% after vaccination.
Conclusions: Vaccine-induced measles and rubella antibody responses are not negatively affected by concomitant administration of HRV. The HRV dose increases antirotavirus serum antibody titers and the proportion of infants with detectable antirotavirus antibody.
Clinical trials registration: NCT01700621.
Keywords: measles vaccine; non-interference; rotavirus vaccine; rubella vaccine; vaccine co-administration.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Can Changes to Scheduling Enhance the Performance of Rotavirus Vaccines in Low-Income Countries?J Infect Dis. 2016 Jun 1;213(11):1673-5. doi: 10.1093/infdis/jiw026. Epub 2016 Jan 27. J Infect Dis. 2016. PMID: 26823336 No abstract available.
References
-
- Kotloff KL, Nataro JP, Blackwelder WC et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. - PubMed
-
- Tate JE, Burton AH, Boschi-Pinto C et al. . 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet ID 2012; 12:136–41. - PubMed
-
- Zaman K, Yunus M, Faruque ASG et al. . Surveillance of rotavirus in a rural diarrhoea treatment centre in Bangladesh, 2000–2006. Vaccine 2009; 27(suppl 5):F31–4. - PubMed
-
- Madhi SA, Cunliffe NA, Steele AD et al. . Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. - PubMed
-
- Zaman K, Dang DA, Victor JC et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

