Long-term outcome after topical ciclosporin in severe dry eye disease with a 10-year follow-up
- PMID: 26823393
- PMCID: PMC5136692
- DOI: 10.1136/bjophthalmol-2015-306930
Long-term outcome after topical ciclosporin in severe dry eye disease with a 10-year follow-up
Abstract
Aim: To report a 10-year follow-up of patients suffering from severe dry eye syndrome (DES) initially treated with topical ciclosporin A (tCSA) for 6 months.
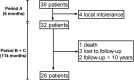
Methods: The charts of 26 patients with severe DES related to keratoconjunctivitis sicca (KCS) and followed for a minimum 10-year follow-up were retrospectively reviewed. All of them were treated initially with tCSA for 6 months. The Schirmer I test, fluorescein and lissamine green staining scores and tear film break-up time (TBUT) were recorded to assess clinical symptoms before, during and after treatment. The subjective signs were evaluated with the ocular surface disease index (OSDI) questionnaire. Prolongation and reintroduction of tCSA after the initial treatment and combined treatments were also noted.
Results: Overall the median (IQR) duration of tCSA treatment was 23 (7-51) months after a prolonged induction treatment lasting 20 (8-41) months during the 10-year follow-up. For symptoms, a statistically significant difference in the OSDI between baseline and the end of the 10-year follow-up was not found (p=0.67). We noted a statistically significant improvement in all clinical signs after the initial treatment period, still present at the end of follow-up. Only 6.5% of the patients needed reintroduction of tCSA after their prolonged induction treatment.
Conclusions: The improvement observed after an initial tCSA treatment was sustained after a long-term follow-up with few cases requiring additional tCSA treatment. A prolonged induction treatment to decrease initial inflammatory local signs is a promising option in KCS.
Keywords: Inflammation; Ocular surface; Tears.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures
References
-
- Lemp MA. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J 1995;21:221–32. - PubMed
-
- Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care 2008;14:S102–6. - PubMed
-
- Yüksel B, Bozdağ B, Acar M, et al. . Evaluation of the effect of topical cyclosporine A with impression cytology in dry eye patients. Eur J Ophthalmol 2010;20:675–9. - PubMed
-
- Sall K, Stevenson OD, Mundorf TK, et al. . Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000;107:631–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources