Multiple Plasmodium falciparum Merozoite Surface Protein 1 Complexes Mediate Merozoite Binding to Human Erythrocytes
- PMID: 26823464
- PMCID: PMC4817195
- DOI: 10.1074/jbc.M115.698282
Multiple Plasmodium falciparum Merozoite Surface Protein 1 Complexes Mediate Merozoite Binding to Human Erythrocytes
Abstract
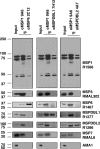
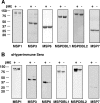
Successful invasion of human erythrocytes byPlasmodium falciparummerozoites is required for infection of the host and parasite survival. The early stages of invasion are mediated via merozoite surface proteins that interact with human erythrocytes. The nature of these interactions are currently not well understood, but it is known that merozoite surface protein 1 (MSP1) is critical for successful erythrocyte invasion. Here we show that the peripheral merozoite surface proteins MSP3, MSP6, MSPDBL1, MSPDBL2, and MSP7 bind directly to MSP1, but independently of each other, to form multiple forms of the MSP1 complex on the parasite surface. These complexes have overlapping functions that interact directly with human erythrocytes. We also show that targeting the p83 fragment of MSP1 using inhibitory antibodies inhibits all forms of MSP1 complexes and disrupts parasite growthin vitro.
Keywords: erythrocyte; infectious disease; invasion; malaria; protein complex.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







Similar articles
-
The merozoite surface protein 1 complex is a platform for binding to human erythrocytes by Plasmodium falciparum.J Biol Chem. 2014 Sep 12;289(37):25655-69. doi: 10.1074/jbc.M114.586495. Epub 2014 Jul 29. J Biol Chem. 2014. PMID: 25074930 Free PMC article.
-
Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes.Eukaryot Cell. 2008 Dec;7(12):2123-32. doi: 10.1128/EC.00274-08. Epub 2008 Sep 26. Eukaryot Cell. 2008. PMID: 18820076 Free PMC article.
-
Proteome analysis reveals a large merozoite surface protein-1 associated complex on the Plasmodium falciparum merozoite surface.J Proteome Res. 2011 Feb 4;10(2):680-91. doi: 10.1021/pr100875y. Epub 2010 Dec 22. J Proteome Res. 2011. PMID: 21175202
-
Merozoite surface proteins of the malaria parasite: the MSP1 complex and the MSP7 family.Int J Parasitol. 2010 Aug 15;40(10):1155-61. doi: 10.1016/j.ijpara.2010.04.008. Epub 2010 May 6. Int J Parasitol. 2010. PMID: 20451527 Review.
-
Erythrocyte invasion receptors for Plasmodium falciparum: new and old.Transfus Med. 2016 Apr;26(2):77-88. doi: 10.1111/tme.12280. Epub 2016 Feb 9. Transfus Med. 2016. PMID: 26862042 Review.
Cited by
-
Plasmodium falciparum merozoite surface protein 3 as a vaccine candidate: a brief review.Rev Inst Med Trop Sao Paulo. 2022 Mar 11;64:e23. doi: 10.1590/S1678-9946202264023. eCollection 2022. Rev Inst Med Trop Sao Paulo. 2022. PMID: 35293561 Free PMC article. Review.
-
Diversity analysis of MSP1 identifies conserved epitope organization in block 2 amidst high sequence variability in Indian Plasmodium falciparum isolates.Malar J. 2018 Dec 3;17(1):447. doi: 10.1186/s12936-018-2592-y. Malar J. 2018. PMID: 30509263 Free PMC article.
-
Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts.Mar Drugs. 2021 Nov 12;19(11):637. doi: 10.3390/md19110637. Mar Drugs. 2021. PMID: 34822508 Free PMC article. Review.
-
Malian field isolates provide insight into Plasmodium malariae intra-erythrocytic development and invasion.PLoS Negl Trop Dis. 2025 Jan 6;19(1):e0012790. doi: 10.1371/journal.pntd.0012790. eCollection 2025 Jan. PLoS Negl Trop Dis. 2025. PMID: 39761327 Free PMC article.
-
A Hetero-Multimeric Chitinase-Containing Plasmodium falciparum and Plasmodium gallinaceum Ookinete-Secreted Protein Complex Involved in Mosquito Midgut Invasion.Front Cell Infect Microbiol. 2021 Jan 8;10:615343. doi: 10.3389/fcimb.2020.615343. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33489941 Free PMC article.
References
-
- Cowman A. F., and Crabb B. S. (2006) Invasion of red blood cells by malaria parasites. Cell 124, 755–766 - PubMed
-
- Gilson P. R., Nebl T., Vukcevic D., Moritz R. L., Sargeant T., Speed T. P., Schofield L., and Crabb B. S. (2006) Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol. Cell. Proteomics 5, 1286–1299 - PubMed
-
- McBride J. S., and Heidrich H. G. (1987) Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23, 71–84 - PubMed
-
- Holder A. A., Sandhu J. S., Hillman Y., Davey L. S., Nicholls S. C., Cooper H., and Lockyer M. J. (1987) Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology 94, 199–208 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

