Recording and labeling at a site along the cochlea shows alignment of medial olivocochlear and auditory nerve tonotopic mappings
- PMID: 26823515
- PMCID: PMC4808113
- DOI: 10.1152/jn.00842.2015
Recording and labeling at a site along the cochlea shows alignment of medial olivocochlear and auditory nerve tonotopic mappings
Abstract
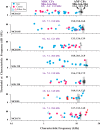
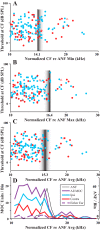
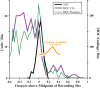
Medial olivocochlear (MOC) neurons provide an efferent innervation to outer hair cells (OHCs) of the cochlea, but their tonotopic mapping is incompletely known. In the present study of anesthetized guinea pigs, the MOC mapping was investigated using in vivo, extracellular recording, and labeling at a site along the cochlear course of the axons. The MOC axons enter the cochlea at its base and spiral apically, successively turning out to innervate OHCs according to their characteristic frequencies (CFs). Recordings made at a site in the cochlear basal turn yielded a distribution of MOC CFs with an upper limit, or "edge," due to usually absent higher-CF axons that presumably innervate more basal locations. The CFs at the edge, normalized across preparations, were equal to the CFs of the auditory nerve fibers (ANFs) at the recording sites (near 16 kHz). Corresponding anatomical data from extracellular injections showed spiraling MOC axons giving rise to an edge of labeling at the position of a narrow band of labeled ANFs. Overall, the edges of the MOC CFs and labeling, with their correspondences to ANFs, suggest similar tonotopic mappings of these efferent and afferent fibers, at least in the cochlear basal turn. They also suggest that MOC axons miss much of the position of the more basally located cochlear amplifier appropriate for their CF; instead, the MOC innervation may be optimized for protection from damage by acoustic overstimulation.
Keywords: afferent; characteristic frequency; cochlear amplifier; efferent; outer hair cell.
Copyright © 2016 the American Physiological Society.
Figures






References
-
- Adams JC. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience 2: 141–145, 1977. - PubMed
-
- Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40: 93–110, 1989. - PubMed
-
- Brown MC. Morphology of labeled afferent fibers in the guinea pig cochlea. J Comp Neurol 260: 591–604, 1987a. - PubMed
-
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol 260: 605–618, 1987b. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

