Tubular Epithelial NF-κB Activity Regulates Ischemic AKI
- PMID: 26823548
- PMCID: PMC5004652
- DOI: 10.1681/ASN.2015070748
Tubular Epithelial NF-κB Activity Regulates Ischemic AKI
Abstract
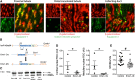
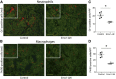
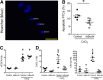
NF-κB is a key regulator of innate and adaptive immunity and is implicated in the pathogenesis of AKI. The cell type-specific functions of NF-κB in the kidney are unknown; however, the pathway serves distinct functions in immune and tissue parenchymal cells. We analyzed tubular epithelial-specific NF-κB signaling in a mouse model of ischemia-reperfusion injury (IRI)-induced AKI. NF-κB reporter activity and nuclear localization of phosphorylated NF-κB subunit p65 analyses in mice revealed that IRI induced widespread NF-κB activation in renal tubular epithelia and in interstitial cells that peaked 2-3 days after injury. To genetically antagonize tubular epithelial NF-κB activity, we generated mice expressing the human NF-κB super-repressor IκBαΔN in renal proximal, distal, and collecting duct epithelial cells. Compared with control mice, these mice exhibited improved renal function, reduced tubular apoptosis, and attenuated neutrophil and macrophage infiltration after IRI-induced AKI. Furthermore, tubular NF-κB-dependent gene expression profiles revealed temporally distinct functional gene clusters for apoptosis, chemotaxis, and morphogenesis. Primary proximal tubular cells isolated from IκBαΔN-expressing mice and exposed to hypoxia-mimetic agent cobalt chloride exhibited less apoptosis and expressed lower levels of chemokines than cells from control mice did. Our results indicate that postischemic NF-κB activation in renal tubular epithelia aggravates tubular injury and exacerbates a maladaptive inflammatory response.
Keywords: NF-kappaB; acute renal failure; apoptosis; chemokine; epithelial cells; ischemia-reperfusion; renal tubular.
Copyright © 2016 by the American Society of Nephrology.
Figures




References
-
- Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G: International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 385: 2616–2643, 2015 - PubMed
-
- Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 - PubMed
-
- Glodowski SD, Wagener G: New insights into the mechanisms of acute kidney injury in the intensive care unit. J Clin Anesth 27: 175–180, 2015 - PubMed
-
- Hinz M, Arslan SC, Scheidereit C: It takes two to tango: IκBs, the multifunctional partners of NF-κB. Immunol Rev 246: 59–76, 2012 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

