Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas
- PMID: 26823601
- PMCID: PMC7831150
- DOI: 10.1158/1078-0432.CCR-15-2550
Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas
Abstract
Purpose: Patients with metastatic or relapsed pediatric sarcomas receive cytotoxic regimens that induce high remission rates associated with profound lymphocyte depletion, but ultimately few survive long term. We administered adjuvant immunotherapy to patients with metastatic and recurrent pediatric sarcomas in an effort to improve outcomes.
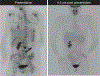
Experimental design: Mononuclear cells were collected via apheresis, and tumor lysate was acquired via percutaneous biopsy at enrollment. Participants received standard antineoplastic therapy, followed by autologous lymphocytes, tumor lysate/keyhole limpet hemocyanin-pulsed dendritic cell vaccinations ± recombinant human IL7. Primary outcomes were toxicity and vaccine responses. Secondary outcomes were immune reconstitution, event-free survival, and overall survival (OS).
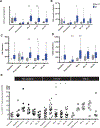
Results: Forty-three patients enrolled and 29 received immunotherapy. The regimen was well tolerated. Intent-to-treat analysis demonstrated 5-year OS of 51% with significant differences based upon histologic group (63% vs. 0% for Ewing/rhabdomyosarcoma vs. other sarcomas) and response to standard therapy (74% no residual disease vs. 0% residual disease). Five-year intent-to-treat OS of patients with newly diagnosed metastatic Ewing/rhabdomyosarcoma was 77%, higher than previously reported in this population and higher than observed in a similar group treated with an earlier adjuvant immunotherapy regimen (25% 5-year OS). T-cell responses to autologous tumor lysate were identified in 62% of immunotherapy recipients, and survival was higher in those patients (73% 5-year OS with vs. 37% without immune response, P = 0.017). Immune reconstitution, measured by CD4 count recovery, was significantly enhanced in subjects treated with recombinant human IL7.
Conclusions: Adjuvant immunotherapy may improve survival in patients with metastatic pediatric sarcoma. Clin Cancer Res; 22(13); 3182-91. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures

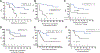
References
-
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694–701. - PubMed
-
- Potratz J, Dirksen U, Jurgens H, Craft A. Ewing sarcoma: clinical state-of-the-art. Pediatr Hematol Oncol 2012;29:1–11. - PubMed
-
- Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with non-metastatic disease. J Clin Oncol 2001;19:3091–102. - PubMed
-
- Stevens MC, Rey A, Bouvet N, Ellershaw C, Flamant F, Habrand JL, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology-SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol 2005; 23:2618–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

