Insulin-like growth factor axis in pregnancies affected by fetal growth disorders
- PMID: 26823688
- PMCID: PMC4730659
- DOI: 10.1186/s13148-016-0178-5
Insulin-like growth factor axis in pregnancies affected by fetal growth disorders
Abstract
Background: Insulin-like growth factors 1 and 2 (IGF1 and IGF2) and their binding proteins (IGFBPs) are expressed in the placenta and known to regulate fetal growth. DNA methylation is an epigenetic mechanism which involves addition of methyl group to a cytosine base in the DNA forming a methylated cytosine-phosphate-guanine (CpG) dinucleotide which is known to silence gene expression. This silences gene expression, potentially altering the expression of IGFs and their binding proteins. This study investigates the relationship between DNA methylation of components of the IGF axis in the placenta and disorders in fetal growth. Placental samples were obtained from cord insertions immediately after delivery from appropriate, small (defined as birthweight <10th percentile for the gestation [SGA]) and macrosomic (defined as birthweight > the 90th percentile for the gestation [LGA]) neonates. Placental DNA methylation, mRNA expression and protein levels of components of the IGF axis were determined by pyrosequencing, rtPCR and Western blotting.
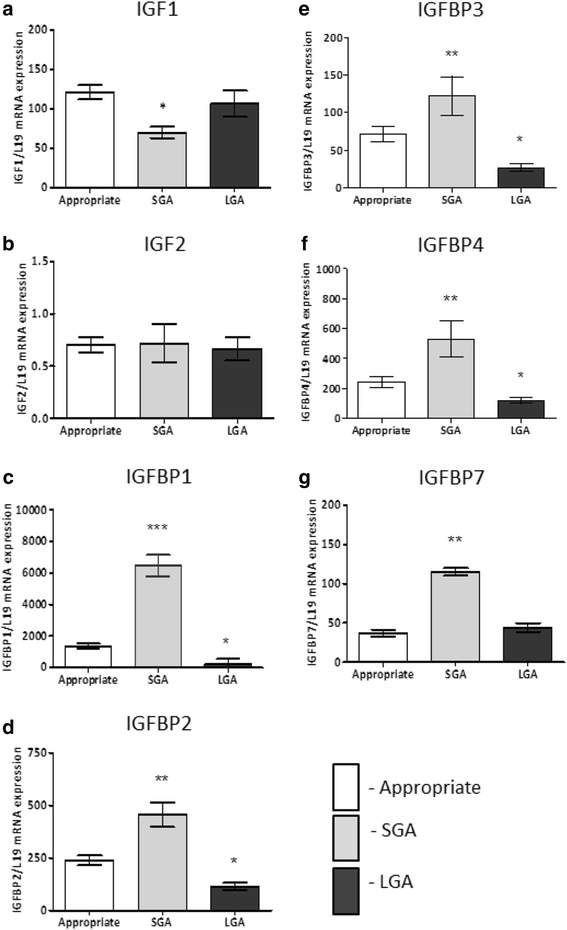
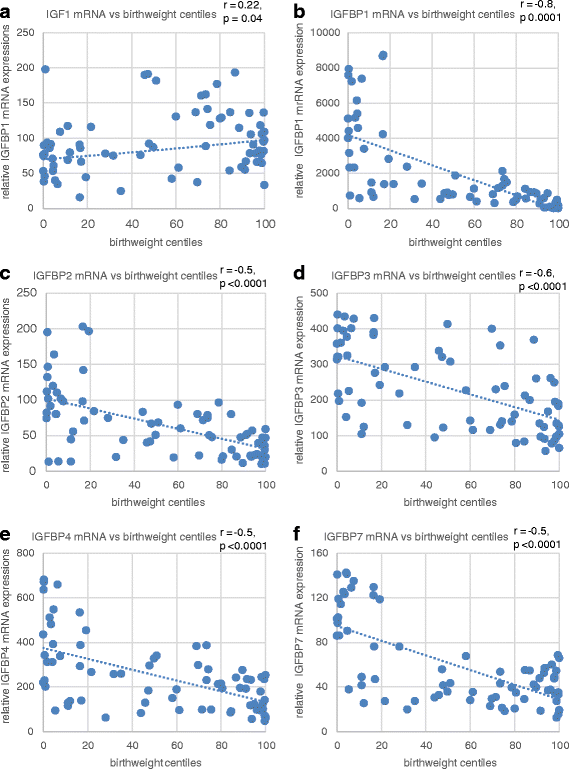
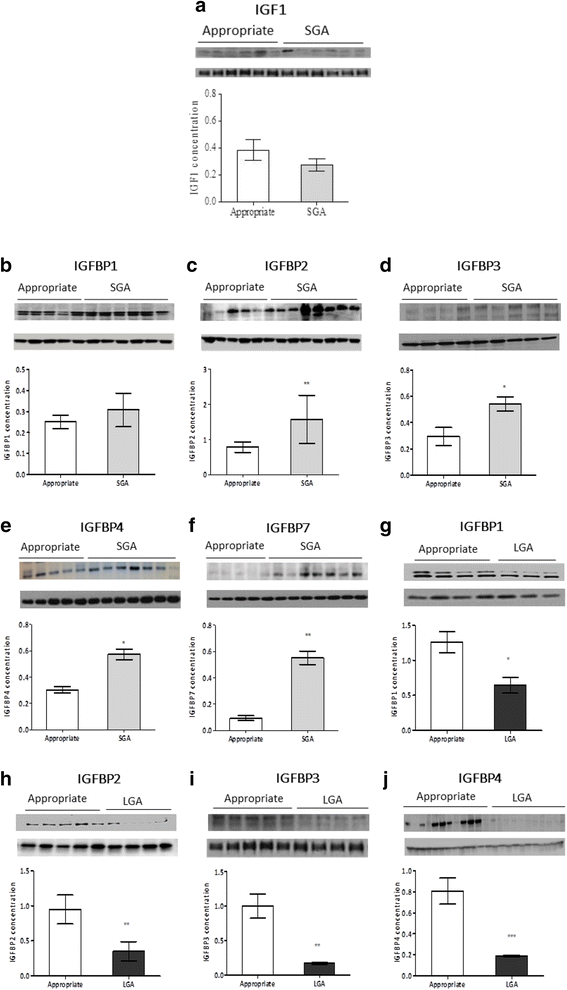
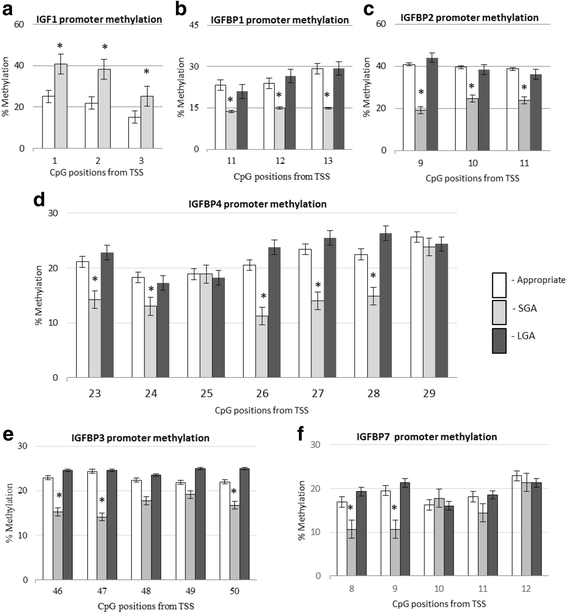
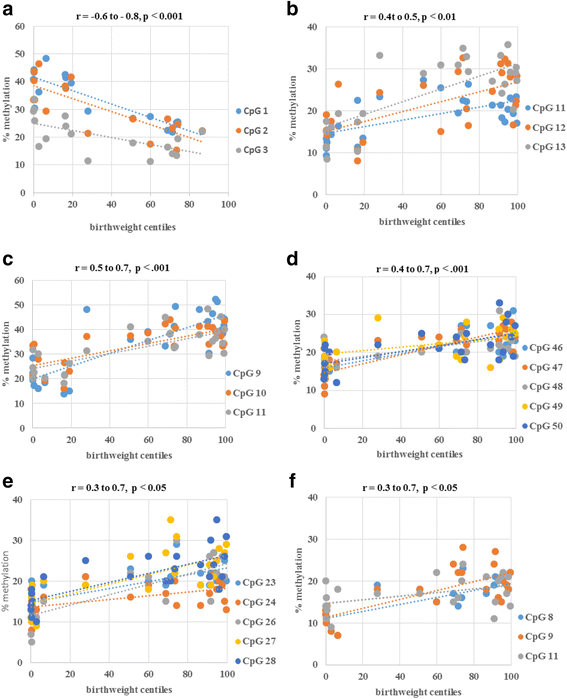
Results: In the placenta from small for gestational age (SGA) neonates (n = 16), mRNA and protein levels of IGF1 were lower and of IGFBPs (1, 2, 3, 4 and 7) were higher (p < 0.05) compared to appropriately grown neonates (n = 37). In contrast, in the placenta from large for gestational age (LGA) neonates (n = 20), mRNA and protein levels of IGF1 was not different and those of IGFBPs (1, 2, 3 and 4) were lower (p < 0.05) compared to appropriately grown neonates. Compared to appropriately grown neonates, CpG methylation of the promoter regions of IGF1 was higher in SGA neonates. The CpG methylation of the promoter regions of IGFBP1, IGFBP2, IGFBP3, IGFBP4 and IGFBP7 was lower in the placenta from SGA neonates as compared to appropriately grown neonates, but was unchanged in the placenta from LGA neonates.
Conclusions: Our results suggest that changes in CpG methylation contribute to the changes in gene expression of components of the IGF axis in fetal growth disorders. Differential methylation of the IGF1 gene and its binding proteins is likely to play a role in the pathogenesis of SGA neonates.
Keywords: DNA methylation; Insulin growth factor; Large fetus; Placental expression; Small fetus.
Figures
Similar articles
-
The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface.J Clin Endocrinol Metab. 1996 Jul;81(7):2680-93. doi: 10.1210/jcem.81.7.8675597. J Clin Endocrinol Metab. 1996. PMID: 8675597
-
The effect of intermittent umbilical cord occlusion on insulin-like growth factors and their binding proteins in preterm and near-term ovine fetuses.J Endocrinol. 2000 Sep;166(3):565-77. doi: 10.1677/joe.0.1660565. J Endocrinol. 2000. PMID: 10974651
-
Expression of messenger RNA of insulin-like growth factors (IGFs) and IGF binding proteins (IGFBP1-6) in placenta of normal and diabetic pregnancy.Endocr J. 1996 Oct;43 Suppl:S89-91. doi: 10.1507/endocrj.43.suppl_s89. Endocr J. 1996. PMID: 9076350 No abstract available.
-
The insulin-like growth factor system and fetal growth restrictionn.Pediatr Endocrinol Rev. 2008 Dec;6(2):235-40. Pediatr Endocrinol Rev. 2008. PMID: 19202510 Review.
-
Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals.Placenta. 2000 May;21(4):289-305. doi: 10.1053/plac.1999.0498. Placenta. 2000. PMID: 10833363 Review.
Cited by
-
Beta-Sitosterol Promotes Milk Protein and Fat Syntheses-Related Genes in Bovine Mammary Epithelial Cells.Animals (Basel). 2021 Nov 12;11(11):3238. doi: 10.3390/ani11113238. Animals (Basel). 2021. PMID: 34827970 Free PMC article.
-
Postnatal Growth Restriction in Mice Alters Cardiac Protein Composition and Leads to Functional Impairment in Adulthood.Int J Mol Sci. 2020 Dec 12;21(24):9459. doi: 10.3390/ijms21249459. Int J Mol Sci. 2020. PMID: 33322681 Free PMC article.
-
Potential Role of Insulin Growth-Factor-Binding Protein 2 as Therapeutic Target for Obesity-Related Insulin Resistance.Int J Mol Sci. 2021 Jan 24;22(3):1133. doi: 10.3390/ijms22031133. Int J Mol Sci. 2021. PMID: 33498859 Free PMC article. Review.
-
Small for Gestational Age Preterm Neonates Exhibit Defective GH/IGF1 Signaling Pathway.Front Pediatr. 2021 Aug 10;9:711400. doi: 10.3389/fped.2021.711400. eCollection 2021. Front Pediatr. 2021. PMID: 34447729 Free PMC article.
-
Placental secretome characterization identifies candidates for pregnancy complications.Commun Biol. 2021 Jun 8;4(1):701. doi: 10.1038/s42003-021-02214-x. Commun Biol. 2021. PMID: 34103657 Free PMC article.
References
-
- Han VK, Bassett N, Walton J, Challis JR. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab. 1996;81(7):2680–93. - PubMed
-
- Takeda Y, Iwashita M. Role of growth factors on fetal growth and maturation. Ann Acad Med Singapore. 1993;22(2):134–41. - PubMed
-
- Hill DJ, Crace CJ, Strain AJ, Milner RD. Regulation of amino acid uptake and deoxyribonucleic acid synthesis in isolated human fetal fibroblasts and myoblasts: effect of human placental lactogen, somatomedin-C, multiplication-stimulating activity, and insulin. J Clin Endocrinol Metab. 1986;62(4):753–60. doi: 10.1210/jcem-62-4-753. - DOI - PubMed
-
- Börzsönyi B, Demendi C, Nagy Z, Tóth K, Csanád M, Pajor A, et al. Gene expression patterns of insulin-like growth factor 1, insulin-like growth factor 2 and insulin-like growth factor binding protein 3 in human placenta from pregnancies with intrauterine growth restriction. J Perinat Med. 2011;39(6):701–7. doi: 10.1515/jpm.2011.090. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

