Roles of Cx43 and AKAP95 in ovarian cancer tissues in G1/S phase
- PMID: 26823747
- PMCID: PMC4713533
Roles of Cx43 and AKAP95 in ovarian cancer tissues in G1/S phase
Abstract
Objective: The purpose of this study was to investigate the expression of A-kinase anchor protein 95 (AKAP95), cell cycle protein E1 (cyclinE1) and D1 (cyclinD1), and gap junction protein connexin 43 (Cx43) in ovarian cancer tissues, the relationship between four proteins and clinicopathologic parameters, and the correlation between these proteins.
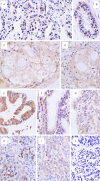
Methods: The expression of proteins in 54 cases of ovarian cancer tissues was detected by immunohistochemical method.
Results: The positive expression rates of AKAP95, cyclinD1 and cyclinE1 in ovarian cancer tissues were 72.22%, 66.67% and 79.63%, respectively, which were higher than that of ovarian pericarcinoma tissues expressing as 33.33%, 25% and 8.30% (P<0.05). The positive expression rate of Cx43 in ovarian cancer tissues was 40.74%, which was lower than that of ovarian pericarcinoma tissues expressing as 75%; respectively, and the difference was statistically significant between groups (P<0.05). The expression of cyclinD1 in ovarian cancer tissues was related to the histologic type (P<0.05) while it showed no correlation with the degree of differentiation (P>0.05). Additionally, the expression of AKAP95, Cx43 and cyclinE1 in ovarian cancer tissues showed no correlation with the degree of differentiation or the histologic type (P>0.05). Protein expressions of AKAP95, Cx43 and cyclinE1 were correlated with each other (P<0.05), and the expressions of cyclinD1, cyclinE1 and Cx43 were also correlated with each other (P<0.05). However, AKAP95 and cyclinD1 showed no correlation (P>0.05).
Conclusion: AKAP95, cyclinD1 and cyclinE1 play an important role in promoting the process of ovarian cancer formation. The tumor inhibitory effects of Cx43 protein on the pathogenesis of ovarian cancer were weakened. The expression of cyclinD1 in ovarian cancer tissues is related to the histologic type while it shows no correlation with the degree of differentiation. Additionally, the expression of AKAP95, Cx43 and cyclinE1 in ovarian cancer tissues shows no correlation with the degree of differentiation or the histologic type. AKAP95 expression is correlated with Cx43 and cyclinE1 expression; Cx43 expression is correlated with AKAP95, cyclinD1 and cyclinE1 expression; cyclinE1 expression is correlated with AKAP95, Cx43, cyclinD1 expression; cyclinD1 expression is correlated with Cx43 and cyclinE1 expression, while AKAP95 and cyclinD1 show no correlation.
Keywords: AKAP95; Cx43; correlation; cyclinD1; cyclinE1; ovarian cancer.
Figures



Similar articles
-
Synergistic effects of AKAP95, Cyclin D1, Cyclin E1, and Cx43 in the development of rectal cancer.Int J Clin Exp Pathol. 2015 Feb 1;8(2):1666-73. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 25973052 Free PMC article.
-
Expression of AKAP95, Cx43, CyclinE1 and CyclinD1 in esophageal cancer and their association with the clinical and pathological parameters.Int J Clin Exp Med. 2015 May 15;8(5):7324-32. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26221272 Free PMC article.
-
Cx43 and AKAP95 regulate G1/S conversion by competitively binding to cyclin E1/E2 in lung cancer cells.Thorac Cancer. 2020 Jun;11(6):1594-1602. doi: 10.1111/1759-7714.13435. Epub 2020 Apr 27. Thorac Cancer. 2020. PMID: 32338437 Free PMC article.
-
[Expression of A-kinase anchor protein 95, cyclin E2, and connexin 43 in lung cancer tissue, clinical significance of their expression, and their expression correlation].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012 Oct;30(10):725-9. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012. PMID: 23256994 Chinese.
-
Epac1, PDE4, and PKC protein expression and their association with AKAP95, Cx43, and cyclinD2/E1 in breast cancer tissues.Thorac Cancer. 2017 Sep;8(5):495-500. doi: 10.1111/1759-7714.12475. Epub 2017 Jul 29. Thorac Cancer. 2017. PMID: 28755423 Free PMC article.
Cited by
-
A-Kinase Anchor Protein 95 Is Involved in ERK1/2-Elk-1 Signal Transduction in Colon Cancer.Anal Cell Pathol (Amst). 2023 Jan 14;2023:8242646. doi: 10.1155/2023/8242646. eCollection 2023. Anal Cell Pathol (Amst). 2023. PMID: 36691407 Free PMC article.
-
Correlations of the expression of Cx43, SCFFBXW7, p-cyclin E1 (Ser73), p-cyclin E1 (Thr77) and p-cyclin E1 (Thr395) in colon cancer tissues.World J Gastrointest Oncol. 2025 Jan 15;17(1):98410. doi: 10.4251/wjgo.v17.i1.98410. World J Gastrointest Oncol. 2025. PMID: 39817129 Free PMC article.
-
Oncogenic splicing regulated by phase separation.Nat Cell Biol. 2020 Aug;22(8):916-918. doi: 10.1038/s41556-020-0553-5. Nat Cell Biol. 2020. PMID: 32719552 No abstract available.
-
Tac2-N serves an oncogenic role and promotes drug resistance in human gastric cancer cells.Exp Ther Med. 2020 Nov;20(5):113. doi: 10.3892/etm.2020.9241. Epub 2020 Sep 18. Exp Ther Med. 2020. PMID: 32989391 Free PMC article.
-
Osthole Alleviates Neointimal Hyperplasia in Balloon-Induced Arterial Wall Injury by Suppressing Vascular Smooth Muscle Cell Proliferation and Downregulating Cyclin D1/CDK4 and Cyclin E1/CDK2 Expression.Front Physiol. 2021 Jan 26;11:514494. doi: 10.3389/fphys.2020.514494. eCollection 2020. Front Physiol. 2021. PMID: 33574763 Free PMC article.
References
-
- Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. CyclinD as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. - PubMed
-
- Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. - PubMed
-
- Arsenijevic T, Degraef C, Dumont JE, Roger PP, PirsonI I. G1/S Cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle. 2006;5:1217–1222. - PubMed
-
- Zhang YW, Nakayama K, Nakayama K, Morita I. A novel route for Connexin 43 to inhibit cell proliferation: negative regulation of S-phase kinase-associated protein (skp2) Cancer Res. 2003;63:1623–1630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
