High-fat diet impairs spatial memory and hippocampal intrinsic excitability and sex-dependently alters circulating insulin and hippocampal insulin sensitivity
- PMID: 26823968
- PMCID: PMC4730722
- DOI: 10.1186/s13293-016-0060-3
High-fat diet impairs spatial memory and hippocampal intrinsic excitability and sex-dependently alters circulating insulin and hippocampal insulin sensitivity
Abstract
Background: High-fat diets promoting obesity/type-2 diabetes can impair physiology and cognitive performance, although sex-dependent comparisons of these impairments are rarely made. Transient reductions in Ca(2+)-dependent afterhyperpolarizations (AHPs) occur during memory consolidation, enhancing intrinsic excitability of hippocampal CA1 pyramidal neurons. In rats fed standard diets, insulin can enhance memory and reduce amplitude and duration of AHPs.
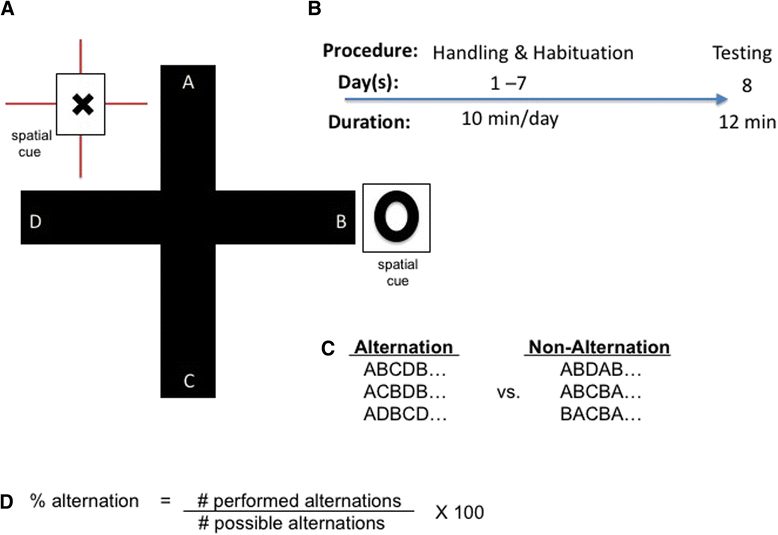
Methods: Effects of chronic high-fat diet (HFD) on memory, circulating insulin, and neuronal physiology were compared between young adult male and female Long-Evans rats. Rats fed for 12 weeks (from weaning) a HFD or a control diet (CD) were then tested in vivo prior to in vitro recordings from CA1 pyramidal neurons.
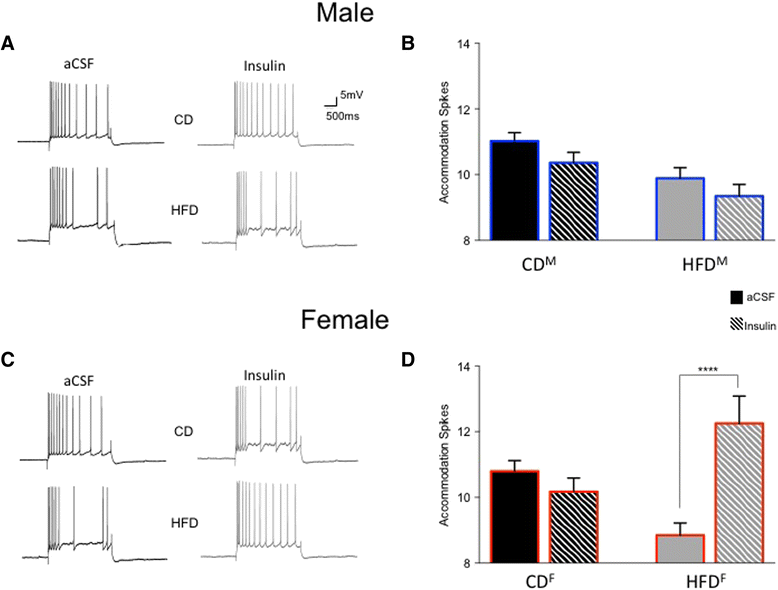
Results: The HFD significantly impaired spatial memory in both males and females. Significant sex differences occurred in circulating insulin and in the insulin sensitivity of hippocampal neurons. Circulating insulin significantly increased in HFD males but decreased in HFD females. While the HFD significantly reduced hippocampal intrinsic excitability in both sexes, CA1 neurons from HFD females remained insulin-sensitive but those from HFD males became insulin-insensitive.
Conclusions: Findings consistent with these have been characterized previously in HFD or senescent males, but the effects observed here in young females are unique. Loss of CA1 neuronal excitability, and sex-dependent loss of insulin sensitivity, can have significant cognitive consequences, over both the short term and the life span. These findings highlight needs for more research into sex-dependent differences, relating systemic and neural plasticity mechanisms in metabolic disorders.
Keywords: AHP; CA1; Diabetes; Glucose regulation; High-fat diet; Hippocampal excitability; Sex differences; Spatial memory.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

