The role of CYP3A5 polymorphism and dose adjustments following conversion of twice-daily to once-daily tacrolimus in renal transplant recipients
- PMID: 26823971
- PMCID: PMC4730664
- DOI: 10.1186/s13737-016-0031-6
The role of CYP3A5 polymorphism and dose adjustments following conversion of twice-daily to once-daily tacrolimus in renal transplant recipients
Abstract
Background: Tacrolimus is available as twice-daily Prograf® (Tac-BID) and the once-daily formulation, Advagraf® (Tac-OD). Although therapeutically equivalent, some transplant recipients require dose adjustments to achieve similar tacrolimus trough concentrations [Tac C0] after conversion between formulations. Tacrolimus is primarily metabolized by cytochrome P450 3A5 (CYP3A5). We sought to determine whether genetic polymorphisms in the CYP3A5 enzyme; CYP3A5 *1/*1 and CYP3A5 *1/*3 (expressers) compared to CYP3A5 *3/*3 (non-expressers) could account for discrepancies in dose requirements following conversion from Tac-BID to Tac-OD.
Methods: A cohort of 60 renal transplant recipients (RTR) from our larger conversion study of 496 patients underwent additional testing for CY3A5 genetic polymorphisms. Analysis included demographics, tac dosing and [Tac C0] pre- and post-conversion and dosing changes relative to CYP3A5 genotypes. CYP3A5 genetic polymorphisms were identified through analysis of genomic DNA.
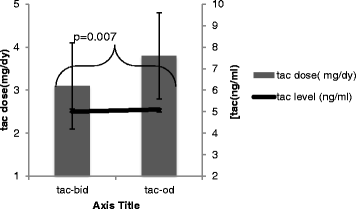
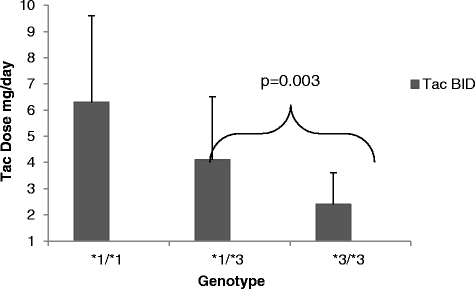
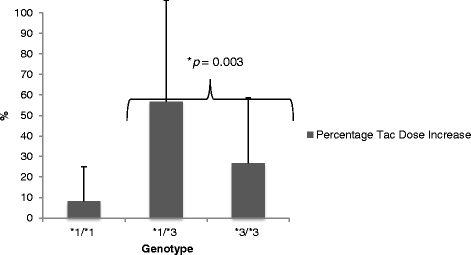
Results: Conversion from tac bid to tac OD in this cohort required a mean (SD) dose increase from 3.1 (1.0) mg/day to 3.8 (1.3) mg/day (p = 0.007), to achieve similar [Tac C0]. The *1/*3 expresser group required a greater percentage dose adjustment (56.7 %) in converting from Tac-BID to Tac-OD as compared to the *3/*3 non-expresser group (26.6 %). Similar findings were observed with the both expresser groups combined (*1/*1 &*1/*3). The expressers were significantly more highly represented in the East Asian cohort.
Conclusions: The CYP3A5 expresser polymorphism necessitates an increase in dosing upon conversion from Tac-BID to Tac-OD, with the expresser genotypes contributing significantly to this finding. Given the variability in frequency of CYP3A5 genotypes in various ethnic groups, future studies should account for both isoenzyme polymorphism and ethnicity in optimizing dosing requirements.
Trial registration: Clinical trials.gov identifier: NCT01884480.
Figures
Similar articles
-
A prospective cohort conversion study of twice-daily to once-daily extended-release tacrolimus: role of ethnicity.Transplant Res. 2014 Mar 10;3(1):7. doi: 10.1186/2047-1440-3-7. Transplant Res. 2014. PMID: 24606676 Free PMC article.
-
Comparative clinical trial of the variability factors of the exposure indices used for the drug monitoring of two tacrolimus formulations in kidney transplant recipients.Pharmacol Res. 2018 Mar;129:84-94. doi: 10.1016/j.phrs.2017.12.005. Epub 2017 Dec 8. Pharmacol Res. 2018. PMID: 29229354 Clinical Trial.
-
Tacrolimus dose adjustment is not necessary in dose to dose conversion from a twice daily to a prolonged release once daily dose form.Sci Rep. 2022 Jun 16;12(1):10051. doi: 10.1038/s41598-022-14317-4. Sci Rep. 2022. PMID: 35710816 Free PMC article.
-
Effects of CPY3A5 Genetic Polymorphisms on the Pharmacokinetics of Extendedrelease and Immediate-release Tacrolimus Formulations in Renal Transplant Recipients: A Systematic Review and Meta-analysis.Curr Drug Metab. 2021;22(10):758-771. doi: 10.2174/1389200222666210825160021. Curr Drug Metab. 2021. PMID: 34525930
-
Effects of CYP3A4*22 polymorphism on trough concentration of tacrolimus in kidney transplantation: a systematic review and meta-analysis.Front Pharmacol. 2023 Jul 26;14:1201083. doi: 10.3389/fphar.2023.1201083. eCollection 2023. Front Pharmacol. 2023. PMID: 37564175 Free PMC article.
Cited by
-
CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment.Pharmgenomics Pers Med. 2018 Mar 7;11:23-33. doi: 10.2147/PGPM.S107710. eCollection 2018. Pharmgenomics Pers Med. 2018. PMID: 29563827 Free PMC article. Review.
References
-
- Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, et al. Two years postconversion from a prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients. Transplantation. 2007;83:1648–1651. doi: 10.1097/01.tp.0000264056.20105.b4. - DOI - PubMed
-
- Min SI, Ha J, Kang HG, Ahn S, Park T, Park DD, et al. Conversion of Twice-Daily Tacrolimus to Once-Daily Tacrolimus Formulation in Stable Pediatric Kidney Transplant Recipients: Pharmacokinetics and Efficacy. American Journal of Transplantation. 2013;13:2191–97. doi: 10.1111/ajt.12274. - DOI - PubMed
-
- Hougardy JM, Broeders N, Kianda M, Massart A, Madhoun P, Le Moine A, et al. Conversion Hustert E, Haberl M, Burk O from Prograf to Advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation. 2011;91:566–569. doi: 10.1097/TP.0b013e3182098ff0. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases