Combined effect of 17β-estradiol and resveratrol against apoptosis induced by interleukin-1β in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway
- PMID: 26824000
- PMCID: PMC4730868
- DOI: 10.7717/peerj.1640
Combined effect of 17β-estradiol and resveratrol against apoptosis induced by interleukin-1β in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway
Abstract
Background: In previous studies, both 17β-estradiol (E2) and resveratrol (RES) were reported to protect intervertebral disc cells against aberrant apoptosis. Given that E2 has a better anti-apoptotic effect with more cancer risk and RES has an anti-apoptotic effect with less cancer risk, the combined use of E2 with RES is promising in developing clinical therapies to treat apoptosis-related diseases such as intervertebral disc degeneration in the future.
Objective: The purpose of this study was to explore the combined effect of E2 with RES on rat nucleus pulposus cells and the underlying mechanisms.
Methods: TUNEL assay and FACS analysis were used to determine apoptotic incidence of nucleus pulposus cells. MTS assay was used to determine cell viability, and cellular binding assay was used to determine cell-ECM (extracellular matrix) ability. Real-time quantitative RT-PCR was to determine mRNA level of target genes. And Western blot was used to determine the protein level.
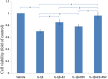
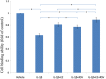
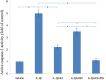
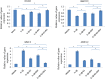
Results: Both E2 and RES decreased apoptotic incidence when used singly; interestingly, they decreased apoptosis more efficiently when used combinedly. Meanwhile, E2 and RES combined together against the decrease of cell viability and binding ability resulting from IL-1β cytotoxicity. As well, activated caspase-3 was suppressed by the combined effect. Furthermore, IL-1β downregulated expression level of type II collagen and aggrecan (standing for anabolism), while upregulated MMP-3 and MMP-13 (standing for catabolism). However, the combined use of E2 with RES effectively abolished the above negative effects caused by IL-1β, better than either single use. Finally, it turned out to be that E2 and RES combined together against apoptosis via the activation of PI3K/Akt/caspase-3 pathway.
Conclusion: This study presented that IL-1β induced aberrant apoptosis, which was efficiently resisted by the combined use of E2 with RES via PI3K/Akt/caspase-3 pathway.
Keywords: 17β-estradiol; Apoptosis; Intervertebral disc degeneration; Nucleus pulposus; PI3K/Akt; Resveratrol.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

