Enzymatically active biomimetic micropropellers for the penetration of mucin gels
- PMID: 26824056
- PMCID: PMC4730841
- DOI: 10.1126/sciadv.1500501
Enzymatically active biomimetic micropropellers for the penetration of mucin gels
Abstract
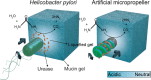
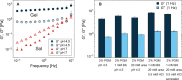
In the body, mucus provides an important defense mechanism by limiting the penetration of pathogens. It is therefore also a major obstacle for the efficient delivery of particle-based drug carriers. The acidic stomach lining in particular is difficult to overcome because mucin glycoproteins form viscoelastic gels under acidic conditions. The bacterium Helicobacter pylori has developed a strategy to overcome the mucus barrier by producing the enzyme urease, which locally raises the pH and consequently liquefies the mucus. This allows the bacteria to swim through mucus and to reach the epithelial surface. We present an artificial system of reactive magnetic micropropellers that mimic this strategy to move through gastric mucin gels by making use of surface-immobilized urease. The results demonstrate the validity of this biomimetic approach to penetrate biological gels, and show that externally propelled microstructures can actively and reversibly manipulate the physical state of their surroundings, suggesting that such particles could potentially penetrate native mucus.
Keywords: Micropropellers; biomimetic penetration of biological gels; gastric mucus barrier; gel-sol transition; helicobacter pylori swimming mechanism; muco-adhesion; propulsion in viscoelastic media; urease surface immobilization.
Figures



Similar articles
-
The Influence of Mucus Microstructure and Rheology in Helicobacter pylori Infection.Front Immunol. 2013 Oct 10;4:310. doi: 10.3389/fimmu.2013.00310. Front Immunol. 2013. PMID: 24133493 Free PMC article. Review.
-
Helicobacter pylori moves through mucus by reducing mucin viscoelasticity.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14321-6. doi: 10.1073/pnas.0903438106. Epub 2009 Aug 11. Proc Natl Acad Sci U S A. 2009. PMID: 19706518 Free PMC article.
-
Unraveling the Intertwined Effect of pH on Helicobacter pylori Motility and the Microrheology of the Mucin-Based Medium It Swims in.Microorganisms. 2023 Nov 10;11(11):2745. doi: 10.3390/microorganisms11112745. Microorganisms. 2023. PMID: 38004756 Free PMC article.
-
Effect of ammonium ion on the hydrophobic and barrier properties of the gastric mucus gel layer: implications on the role of ammonium in H. pylori-induced gastritis.J Gastroenterol Hepatol. 1994;9 Suppl 1:S13-9. doi: 10.1111/j.1440-1746.1994.tb01295.x. J Gastroenterol Hepatol. 1994. PMID: 7881013
-
The properties of the mucus barrier, a unique gel--how can nanoparticles cross it?Ther Deliv. 2016;7(4):229-44. doi: 10.4155/tde-2015-0002. Ther Deliv. 2016. PMID: 27010985 Review.
Cited by
-
Towards multifunctional robotic pills.Nat Biomed Eng. 2024 Nov;8(11):1334-1346. doi: 10.1038/s41551-023-01090-6. Epub 2023 Sep 18. Nat Biomed Eng. 2024. PMID: 37723325 Review.
-
How safe are magnetic nanomotors: From cells to animals.Biomater Adv. 2022 Sep;140:213048. doi: 10.1016/j.bioadv.2022.213048. Epub 2022 Aug 3. Biomater Adv. 2022. PMID: 35939957 Free PMC article.
-
Biohybrid Microrobots Based on Jellyfish Stinging Capsules and Janus Particles for In Vitro Deep-Tissue Drug Penetration.Small Sci. 2025 Feb 11;5(6):2400551. doi: 10.1002/smsc.202400551. eCollection 2025 Jun. Small Sci. 2025. PMID: 40529856 Free PMC article.
-
Magnetically Driven Micro and Nanorobots.Chem Rev. 2021 Apr 28;121(8):4999-5041. doi: 10.1021/acs.chemrev.0c01234. Epub 2021 Mar 31. Chem Rev. 2021. PMID: 33787235 Free PMC article.
-
Bioinspired urease-powered micromotor as an active oral drug delivery carrier in stomach.Bioact Mater. 2021 Aug 10;9:54-62. doi: 10.1016/j.bioactmat.2021.08.004. eCollection 2022 Mar. Bioact Mater. 2021. PMID: 34820555 Free PMC article.
References
-
- Cone R. A., Barrier properties of mucus. Adv. Drug Deliv. Rev. 61, 75–85 (2009). - PubMed
-
- Celli J. P., Turner B. S., Afdhal N. H., Ewoldt R. H., McKinley G. H., Bansil R., Erramilli S., Rheology of gastric mucin exhibits a pH-dependent sol–gel transition. Biomacromolecules 8, 1580–1586 (2007). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

