Chemical synthesis of erythropoietin glycoforms for insights into the relationship between glycosylation pattern and bioactivity
- PMID: 26824070
- PMCID: PMC4730857
- DOI: 10.1126/sciadv.1500678
Chemical synthesis of erythropoietin glycoforms for insights into the relationship between glycosylation pattern and bioactivity
Abstract
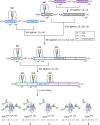
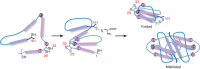
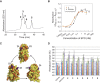
The role of sialyloligosaccharides on the surface of secreted glycoproteins is still unclear because of the difficulty in the preparation of sialylglycoproteins in a homogeneous form. We selected erythropoietin (EPO) as a target molecule and designed an efficient synthetic strategy for the chemical synthesis of a homogeneous form of five EPO glycoforms varying in glycosylation position and the number of human-type biantennary sialyloligosaccharides. A segment coupling strategy performed by native chemical ligation using six peptide segments including glycopeptides yielded homogeneous EPO glycopeptides, and folding experiments of these glycopeptides afforded the correctly folded EPO glycoforms. In an in vivo erythropoiesis assay in mice, all of the EPO glycoforms displayed biological activity, in particular the EPO bearing three sialyloligosaccharides, which exhibited the highest activity. Furthermore, we observed that the hydrophilicity and biological activity of the EPO glycoforms varied depending on the glycosylation pattern. This knowledge will pave the way for the development of homogeneous biologics by chemical synthesis.
Keywords: Biotechnology; chemical glycoprotein synthesis; chemical protein synthesis; glycoprotein; glycosylation; oligosaccharide.
Figures


Similar articles
-
Recent Progress in the Synthesis of Homogeneous Erythropoietin (EPO) Glycoforms.Chembiochem. 2020 Dec 1;21(23):3301-3312. doi: 10.1002/cbic.202000347. Epub 2020 Jul 30. Chembiochem. 2020. PMID: 33210450 Review.
-
Chemical and Enzymatic Synthesis of Sialylated Glycoforms of Human Erythropoietin.Angew Chem Int Ed Engl. 2021 Dec 1;60(49):25922-25932. doi: 10.1002/anie.202110013. Epub 2021 Nov 2. Angew Chem Int Ed Engl. 2021. PMID: 34523784 Free PMC article.
-
Toward homogeneous erythropoietin: non-NCL-based chemical synthesis of the Gln78-Arg166 glycopeptide domain.J Am Chem Soc. 2009 Apr 22;131(15):5424-31. doi: 10.1021/ja808704m. J Am Chem Soc. 2009. PMID: 19334683 Free PMC article.
-
Selective in vitro glycosylation of recombinant proteins: semi-synthesis of novel homogeneous glycoforms of human erythropoietin.Chem Biol. 2001 Feb;8(2):133-45. doi: 10.1016/s1074-5521(00)90065-6. Chem Biol. 2001. PMID: 11251288
-
Chemical synthesis of homogeneous glycopeptides and glycoproteins.Chem Rec. 2010 Apr;10(2):80-100. doi: 10.1002/tcr.200900024. Chem Rec. 2010. PMID: 20349507 Review.
Cited by
-
Chemically Precise Glycoengineering Improves Human Insulin.ACS Chem Biol. 2018 Jan 19;13(1):73-81. doi: 10.1021/acschembio.7b00794. Epub 2017 Dec 1. ACS Chem Biol. 2018. PMID: 29090903 Free PMC article.
-
D-Peptide and D-Protein Technology: Recent Advances, Challenges, and Opportunities.Chembiochem. 2023 Feb 14;24(4):e202200537. doi: 10.1002/cbic.202200537. Epub 2022 Nov 16. Chembiochem. 2023. PMID: 36278392 Free PMC article. Review.
-
Traceless β-mercaptan-assisted activation of valinyl benzimidazolinones in peptide ligations.Chem Sci. 2018 Jan 5;9(7):1940-1946. doi: 10.1039/c7sc04148a. eCollection 2018 Feb 21. Chem Sci. 2018. PMID: 29675240 Free PMC article.
-
Deciphering protein post-translational modifications using chemical biology tools.Nat Rev Chem. 2020 Dec;4(12):674-695. doi: 10.1038/s41570-020-00223-8. Epub 2020 Oct 6. Nat Rev Chem. 2020. PMID: 37127974 Review.
-
A synthetic approach to 'click' neoglycoprotein analogues of EPO employing one-pot native chemical ligation and CuAAC chemistry.Chem Sci. 2018 Oct 29;10(3):815-828. doi: 10.1039/c8sc03409e. eCollection 2019 Jan 21. Chem Sci. 2018. PMID: 30774876 Free PMC article.
References
-
- K. C. Nicolaou, T. Montagnon, Molecules That Changed the World (Wiley-VCH, Weinheim, Germany, 2008).
-
- Lappin T. R., Maxwell A. P., Johnston P. G., EPO’s alter ego: Erythropoietin has multiple actions. Stem Cells 20, 485–492 (2002). - PubMed
-
- Walsh G., Jefferis R., Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 24, 1241–1252 (2006). - PubMed
-
- Dawson P. E., Muir T. W., Clark-Lewis I., Kent S. B., Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994). - PubMed
-
- Gamblin D. P., Scanlan E. M., Davis B. G., Glycoprotein synthesis: An update. Chem. Rev. 109, 131–163 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

