TGFβ-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance
- PMID: 26824266
- PMCID: PMC4749558
- DOI: 10.7554/eLife.08133
TGFβ-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance
Abstract
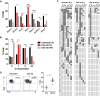
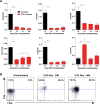
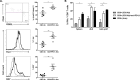
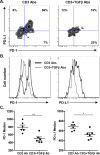
CD8(+) T cell anergy is a critical mechanism of peripheral tolerance, poorly investigated in response to immunotherapy. Here, using a pancreatic islet allograft model and CD3 antibody therapy, we showed, by single cell gene profiling, that intragraft CD8(+) lymphocytes coexpressing granzyme B and perforin were selectively depleted through the Fas/FasL pathway. This step led to long-standing anergy of the remaining CD8(+) T cells marked by the absence of cytotoxic/inflammatory gene expression also confirmed by transcriptome analysis. This sustained unresponsiveness required the presence of the alloantigens. Furthermore, tissue-resident CD8(+) lymphocytes produced TGFβ and expressed the inhibitory receptors PD-1 and PD-L1. Blockade of TGFβ downregulated PD-1 and PD-L1 expression and precipitated graft rejection. Neutralizing PD-1, PD-L1 or TGFβRII signaling in T cells also abrogated CD3 antibody-induced tolerance. These studies unravel novel mechanisms underlying CD8(+) T cell anergy and reveal a cell intrinsic regulatory link between the TGFβ and the PD-1/PD-L1 pathways.
Keywords: CD8+ T cells; PD-1/PD-L1; TGF-beta; anergy; immunology; mouse; tolerance; tranplantation.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures












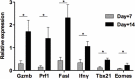

References
-
- Ayroldi E, Migliorati G, Cannarile L, Moraca R, Delfino DV, Riccardi C. CD2 rescues t cells from t-cell receptor/CD3 apoptosis: a role for the Fas/Fas-l system. Blood. 1997;89:3717–3726. - PubMed
-
- Baas MC, Besançon A, Sawitzki B, Mangez C, Valette F, Chatenoud L, You S. Intragraft mechanisms associated with the immunosuppressive versus the tolerogenic effect of CD3 antibodies in a mouse model of islet allografts. Transplantation Proceedings. 2013;45:1895–1898. doi: 10.1016/j.transproceed.2013.01.054. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

