MIIP accelerates epidermal growth factor receptor protein turnover and attenuates proliferation in non-small cell lung cancer
- PMID: 26824318
- PMCID: PMC4891030
- DOI: 10.18632/oncotarget.7001
MIIP accelerates epidermal growth factor receptor protein turnover and attenuates proliferation in non-small cell lung cancer
Abstract
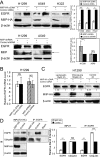
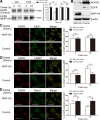
The migration and invasion inhibitory protein (MIIP) has been discovered recently to have inhibitory functions in cell proliferation and migration. Overexpression of MIIP reduced the intracellular steady-state level of epidermal growth factor receptor (EGFR) protein in lung cancer cells with no effect on EGFR mRNA expression compared to that in the control cells. This MIIP-promoted EGFR protein degradation was reversed by proteasome and lysosome inhibitors, suggesting the involvement of both proteasomal and lysosomal pathways in this degradation. This finding was further validated by pulse-chase experiments using 35S-methionine metabolic labeling. We found that MIIP accelerates EGFR protein turnover via proteasomal degradation in the endoplasmic reticulum and then via the lysosomal pathway after its entry into endocytic trafficking. MIIP-stimulated downregulation of EGFR inhibits downstream activation of Ras and blocks the MEK signal transduction pathway, resulting in inhibition of cell proliferation. The negative correlation between MIIP and EGFR protein expression was validated in lung adenocarcinoma samples. Furthermore, the higher MIIP protein expression predicts a better overall survival of Stage IA-IIIA lung adenocarcinoma patients who underwent radical surgery. These findings reveal a new mechanism by which MIIP inhibits cell proliferation.
Keywords: epidermal growth factor receptor; migration and invasion inhibitory protein; non-small cell lung cancer; protein degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Hirsch FR, Varella-Garcia M, Cappuzzo F. Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene. 2009;28(Suppl 1):S32–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

