Tracking Sodium-Antimonide Phase Transformations in Sodium-Ion Anodes: Insights from Operando Pair Distribution Function Analysis and Solid-State NMR Spectroscopy
- PMID: 26824406
- PMCID: PMC4819537
- DOI: 10.1021/jacs.5b13273
Tracking Sodium-Antimonide Phase Transformations in Sodium-Ion Anodes: Insights from Operando Pair Distribution Function Analysis and Solid-State NMR Spectroscopy
Abstract
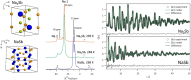
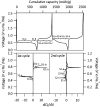
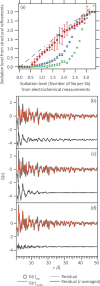
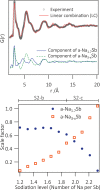
Operando pair distribution function (PDF) analysis and ex situ (23)Na magic-angle spinning solid-state nuclear magnetic resonance (MAS ssNMR) spectroscopy are used to gain insight into the alloying mechanism of high-capacity antimony anodes for sodium-ion batteries. Subtraction of the PDF of crystalline NaxSb phases from the total PDF, an approach constrained by chemical phase information gained from (23)Na ssNMR in reference to relevant model compounds, identifies two previously uncharacterized intermediate species formed electrochemically; a-Na(3-x)Sb (x ≈ 0.4-0.5), a structure locally similar to crystalline Na3Sb (c-Na3Sb) but with significant numbers of sodium vacancies and a limited correlation length, and a-Na(1.7)Sb, a highly amorphous structure featuring some Sb-Sb bonding. The first sodiation breaks down the crystalline antimony to form first a-Na(3-x)Sb and, finally, crystalline Na3Sb. Desodiation results in the formation of an electrode formed of a composite of crystalline and amorphous antimony networks. We link the different reactivity of these networks to a series of sequential sodiation reactions manifesting as a cascade of processes observed in the electrochemical profile of subsequent cycles. The amorphous network reacts at higher voltages reforming a-Na(1.7)Sb, then a-Na(3-x)Sb, whereas lower potentials are required for the sodiation of crystalline antimony, which reacts to form a-Na(3-x)Sb without the formation of a-Na(1.7)Sb. a-Na(3-x)Sb is converted to crystalline Na3Sb at the end of the second discharge. We find no evidence of formation of NaSb. Variable temperature (23)Na NMR experiments reveal significant sodium mobility within c-Na3Sb; this is a possible contributing factor to the excellent rate performance of Sb anodes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Komaba S.; Matsuura Y.; Ishikawa I.; Yabuuchi N.; Murata W.; Kuze S. Electrochem. Commun. 2012, 21, 65.10.1016/j.elecom.2012.05.017. - DOI
-
- Baggetto L.; Keum J. K.; Browning J. F.; Veith G. M. Electrochem. Commun. 2013, 34, 41.10.1016/j.elecom.2013.05.025. - DOI
-
- Baggetto L.; Ganesh P.; Sun C.-N.; Meisner R. A.; Zawodzinski T. A.; Veith G. M. J. Mater. Chem. A 2013, 1, 7985.10.1039/c3ta11568b. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

