A Biophysical Model of CRISPR/Cas9 Activity for Rational Design of Genome Editing and Gene Regulation
- PMID: 26824432
- PMCID: PMC4732943
- DOI: 10.1371/journal.pcbi.1004724
A Biophysical Model of CRISPR/Cas9 Activity for Rational Design of Genome Editing and Gene Regulation
Abstract
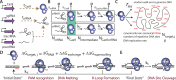
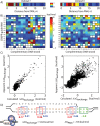
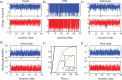
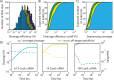
The ability to precisely modify genomes and regulate specific genes will greatly accelerate several medical and engineering applications. The CRISPR/Cas9 (Type II) system binds and cuts DNA using guide RNAs, though the variables that control its on-target and off-target activity remain poorly characterized. Here, we develop and parameterize a system-wide biophysical model of Cas9-based genome editing and gene regulation to predict how changing guide RNA sequences, DNA superhelical densities, Cas9 and crRNA expression levels, organisms and growth conditions, and experimental conditions collectively control the dynamics of dCas9-based binding and Cas9-based cleavage at all DNA sites with both canonical and non-canonical PAMs. We combine statistical thermodynamics and kinetics to model Cas9:crRNA complex formation, diffusion, site selection, reversible R-loop formation, and cleavage, using large amounts of structural, biochemical, expression, and next-generation sequencing data to determine kinetic parameters and develop free energy models. Our results identify DNA supercoiling as a novel mechanism controlling Cas9 binding. Using the model, we predict Cas9 off-target binding frequencies across the lambdaphage and human genomes, and explain why Cas9's off-target activity can be so high. With this improved understanding, we propose several rules for designing experiments for minimizing off-target activity. We also discuss the implications for engineering dCas9-based genetic circuits.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Inhibition Mechanism of an Anti-CRISPR Suppressor AcrIIA4 Targeting SpyCas9.Mol Cell. 2017 Jul 6;67(1):117-127.e5. doi: 10.1016/j.molcel.2017.05.024. Epub 2017 Jun 9. Mol Cell. 2017. PMID: 28602637 Free PMC article.
-
Structural Basis for the Canonical and Non-canonical PAM Recognition by CRISPR-Cpf1.Mol Cell. 2017 Aug 17;67(4):633-645.e3. doi: 10.1016/j.molcel.2017.06.035. Epub 2017 Aug 3. Mol Cell. 2017. PMID: 28781234 Free PMC article.
-
Structural roles of guide RNAs in the nuclease activity of Cas9 endonuclease.Nat Commun. 2016 Nov 2;7:13350. doi: 10.1038/ncomms13350. Nat Commun. 2016. PMID: 27804953 Free PMC article.
-
CRISPR-Cas9 Structures and Mechanisms.Annu Rev Biophys. 2017 May 22;46:505-529. doi: 10.1146/annurev-biophys-062215-010822. Epub 2017 Mar 30. Annu Rev Biophys. 2017. PMID: 28375731 Review.
-
Optimization of genome editing through CRISPR-Cas9 engineering.Bioengineered. 2016 Apr;7(3):166-74. doi: 10.1080/21655979.2016.1189039. Bioengineered. 2016. PMID: 27340770 Free PMC article. Review.
Cited by
-
crRNA complementarity shifts endogenous CRISPR-Cas systems between transcriptional repression and DNA defense.RNA Biol. 2021 Nov;18(11):1560-1573. doi: 10.1080/15476286.2021.1878335. Epub 2021 Mar 18. RNA Biol. 2021. PMID: 33733999 Free PMC article.
-
The significance of bioengineered nanoplatforms against SARS-CoV-2: From detection to genome editing.Life Sci. 2021 Jun 1;274:119289. doi: 10.1016/j.lfs.2021.119289. Epub 2021 Mar 4. Life Sci. 2021. PMID: 33676931 Free PMC article. Review.
-
The novel anti-CRISPR AcrIIA22 relieves DNA torsion in target plasmids and impairs SpyCas9 activity.PLoS Biol. 2021 Oct 13;19(10):e3001428. doi: 10.1371/journal.pbio.3001428. eCollection 2021 Oct. PLoS Biol. 2021. PMID: 34644300 Free PMC article.
-
Predictive design of sigma factor-specific promoters.Nat Commun. 2020 Nov 16;11(1):5822. doi: 10.1038/s41467-020-19446-w. Nat Commun. 2020. PMID: 33199691 Free PMC article.
-
Burden-driven feedback control of gene expression.Nat Methods. 2018 May;15(5):387-393. doi: 10.1038/nmeth.4635. Epub 2018 Mar 26. Nat Methods. 2018. PMID: 29578536
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

