Clinical Evaluation of an Affordable Qualitative Viral Failure Assay for HIV Using Dried Blood Spots in Uganda
- PMID: 26824465
- PMCID: PMC4732948
- DOI: 10.1371/journal.pone.0145110
Clinical Evaluation of an Affordable Qualitative Viral Failure Assay for HIV Using Dried Blood Spots in Uganda
Abstract
Background: WHO recommends regular viral load (VL) monitoring of patients on antiretroviral therapy (ART) for timely detection of virological failure, prevention of acquired HIV drug resistance (HIVDR) and avoiding unnecessary switching to second-line ART. However, the cost and complexity of routine VL testing remains prohibitive in most resource limited settings (RLS). We evaluated a simple, low-cost, qualitative viral-failure assay (VFA) on dried blood spots (DBS) in three clinical settings in Uganda.
Methods: We conducted a cross-sectional diagnostic accuracy study in three HIV/AIDS treatment centres at the Joint Clinical Research Centre in Uganda. The VFA employs semi-quantitative detection of HIV-1 RNA amplified from the LTR gene. We used paired dry blood spot (DBS) and plasma with the COBASAmpliPrep/COBASTaqMan, Roche version 2 (VLref) as the reference assay. We used the VFA at two thresholds of viral load, (>5,000 or >1,000 copies/ml).
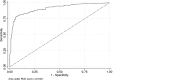
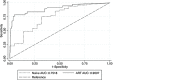
Results: 496 paired VFA and VLref results were available for comparative analysis. Overall, VFA demonstrated 78.4% sensitivity, (95% CI: 69.7%-87.1%), 93% specificity (95% CI: 89.7%-96.4%), 89.3% accuracy (95% CI: 85%-92%) and an agreement kappa = 0.72 as compared to the VLref. The predictive values of positivity and negativity among patients on ART for >12 months were 72.7% and 99.3%, respectively.
Conclusions: VFA allowed 89% of correct classification of VF. Only 11% of the patients were misclassified with the potential of unnecessary or late switch to second-line ART. Our findings present an opportunity to roll out simple and affordable VL monitoring for HIV-1 treatment in RLS.
Conflict of interest statement
Figures
References
-
- UNAIDS (2013) Global Report: UNAIDS Report on the global AIDS epidemic 2013 Geneva, Switzerland: United Nations.
-
- UNAIDS (2015) How AIDS changed everything. MDG 6: 15 years, 15 lessons of hope from the AIDS response. Fact sheet. Geneva, Switzerland: United Nations. Accessed 15.09.2015 at: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf
-
- Wolrd Bank (2013) Gross Domestic Product 2013. The World Bank Group.
-
- WHO (2013) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach Geneva, Switzerland: World Health Organization. - PubMed
-
- Médecins Sans Frontières (2013) How Low Can We Go? Pricing for HIV Viral Load Testing in Low- and Middle-Income Countries. Paris, France: Médecins Sans Frontières; 1–8 p. Accessed 03.04.2015 at: http://www.msf.org/sites/msf.org/files/how_low_can_we_go_vl_pricing_brie...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

