The Early-Acting Peroxin PEX19 Is Redundantly Encoded, Farnesylated, and Essential for Viability in Arabidopsis thaliana
- PMID: 26824478
- PMCID: PMC4733102
- DOI: 10.1371/journal.pone.0148335
The Early-Acting Peroxin PEX19 Is Redundantly Encoded, Farnesylated, and Essential for Viability in Arabidopsis thaliana
Abstract
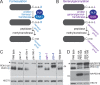
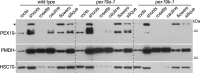
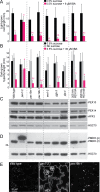
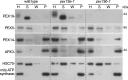
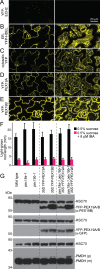
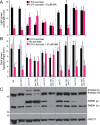
Peroxisomes are single-membrane bound organelles that are essential for normal development in plants and animals. In mammals and yeast, the peroxin (PEX) proteins PEX3 and PEX19 facilitate the early steps of peroxisome membrane protein (PMP) insertion and pre-peroxisome budding from the endoplasmic reticulum. The PEX3 membrane protein acts as a docking site for PEX19, a cytosolic chaperone for PMPs that delivers PMPs to the endoplasmic reticulum or peroxisomal membrane. PEX19 is farnesylated in yeast and mammals, and we used immunoblotting with prenylation mutants to show that PEX19 also is fully farnesylated in wild-type Arabidopsis thaliana plants. We examined insertional alleles disrupting either of the two Arabidopsis PEX19 isoforms, PEX19A or PEX19B, and detected similar levels of PEX19 protein in the pex19a-1 mutant and wild type; however, PEX19 protein was nearly undetectable in the pex19b-1 mutant. Despite the reduction in PEX19 levels in pex19b-1, both pex19a-1 and pex19b-1 single mutants lacked notable peroxisomal β-oxidation defects and displayed normal levels and localization of peroxisomal matrix and membrane proteins. The pex19a-1 pex19b-1 double mutant was embryo lethal, indicating a redundantly encoded critical role for PEX19 during embryogenesis. Expressing YFP-tagged versions of either PEX19 isoform rescued this lethality, confirming that PEX19A and PEX19B act redundantly in Arabidopsis. We observed that pex19b-1 enhanced peroxisome-related defects of a subset of peroxin-defective mutants, supporting a role for PEX19 in peroxisome function. Together, our data indicate that Arabidopsis PEX19 promotes peroxisome function and is essential for viability.
Conflict of interest statement
Figures

References
-
- Zolman BK, Nyberg M, Bartel B. IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Mol Biol. 2007; 64: 59–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

