K+ Channel Inhibition Differentially Regulates Migration of Intestinal Epithelial Cells in Inflamed vs. Non-Inflamed Conditions in a PI3K/Akt-Mediated Manner
- PMID: 26824610
- PMCID: PMC4732808
- DOI: 10.1371/journal.pone.0147736
K+ Channel Inhibition Differentially Regulates Migration of Intestinal Epithelial Cells in Inflamed vs. Non-Inflamed Conditions in a PI3K/Akt-Mediated Manner
Abstract
Background: Potassium channels have been shown to determine wound healing in different tissues, but their role in intestinal epithelial restitution--the rapid closure of superficial wounds by intestinal epithelial cells (IEC)--remains unclear.
Methods: In this study, the regulation of IEC migration by potassium channel modulation was explored with and without additional epidermal growth factor (EGF) under baseline and interferon-γ (IFN-γ)-pretreated conditions in scratch assays and Boyden chamber assays using the intestinal epithelial cell lines IEC-18 and HT-29. To identify possibly involved subcellular pathways, Western Blot (WB)-analysis of ERK and Akt phosphorylation was conducted and PI3K and ERK inhibitors were used in scratch assays. Furthermore, mRNA-levels of the potassium channel KCNN4 were determined in IEC from patients suffering from inflammatory bowel diseases (IBD).
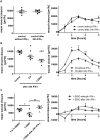
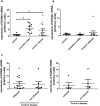
Results: Inhibition of Ca(2+)-dependent potassium channels significantly increased intestinal epithelial restitution, which could not be further promoted by additional EGF. In contrast, inhibition of KCNN4 after pretreatment with IFN-γ led to decreased or unaffected migration. This effect was abolished by EGF. Changes in Akt, but not in ERK phosphorylation strongly correlated with these findings and PI3K but not ERK inhibition abrogated the effect of KCNN4 inhibition. Levels of KCNN4 mRNA were higher in samples from IBD patients compared with controls.
Conclusions: Taken together, we demonstrate that inhibition of KCNN4 differentially regulates IEC migration in IFN-γ-pretreated vs. non pretreated conditions. Moreover, our data propose that the PI3K signaling cascade is responsible for this differential regulation. Therefore, we present a cellular model that contributes new aspects to epithelial barrier dysfunction in chronic intestinal inflammation, resulting in propagation of inflammation and symptoms like ulcers or diarrhea.
Conflict of interest statement
Figures







Similar articles
-
KCNN4 Expression Is Elevated in Inflammatory Bowel Disease: This Might Be a Novel Marker and Therapeutic Option Targeting Potassium Channels.J Gastrointestin Liver Dis. 2020 Dec 12;29(4):539-547. doi: 10.15403/jgld-903. J Gastrointestin Liver Dis. 2020. PMID: 33331347
-
Periodic Mechanical Stress Activates PKCδ-Dependent EGFR Mitogenic Signals in Rat Chondrocytes via PI3K-Akt and ERK1/2.Cell Physiol Biochem. 2016;39(4):1281-94. doi: 10.1159/000447833. Epub 2016 Sep 8. Cell Physiol Biochem. 2016. PMID: 27606614
-
CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity.Am J Physiol Cell Physiol. 2010 Jul;299(1):C175-84. doi: 10.1152/ajpcell.00344.2009. Epub 2010 Apr 14. Am J Physiol Cell Physiol. 2010. PMID: 20392929
-
Epithelial restitution and wound healing in inflammatory bowel disease.World J Gastroenterol. 2008 Jan 21;14(3):348-53. doi: 10.3748/wjg.14.348. World J Gastroenterol. 2008. PMID: 18200658 Free PMC article. Review.
-
Potassium channels in intestinal epithelial cells and their pharmacological modulation: a systematic review.Am J Physiol Cell Physiol. 2021 Apr 1;320(4):C520-C546. doi: 10.1152/ajpcell.00393.2020. Epub 2020 Dec 16. Am J Physiol Cell Physiol. 2021. PMID: 33326312 Free PMC article.
Cited by
-
Histone Deacetylases Enhance Ca2+-Activated K⁺ Channel KCa3.1 Expression in Murine Inflammatory CD4⁺ T Cells.Int J Mol Sci. 2018 Sep 27;19(10):2942. doi: 10.3390/ijms19102942. Int J Mol Sci. 2018. PMID: 30262728 Free PMC article.
-
AgTx2-GFP, Fluorescent Blocker Targeting Pharmacologically Important Kv1.x (x = 1, 3, 6) Channels.Toxins (Basel). 2023 Mar 18;15(3):229. doi: 10.3390/toxins15030229. Toxins (Basel). 2023. PMID: 36977120 Free PMC article.
-
Dynamic Gene Network Analysis of Caco-2 Cell Response to Shiga Toxin-Producing Escherichia coli-Associated Hemolytic-Uremic Syndrome.Microorganisms. 2019 Jul 8;7(7):195. doi: 10.3390/microorganisms7070195. Microorganisms. 2019. PMID: 31288487 Free PMC article.
-
Immune Cell Circuits in Mucosal Wound Healing: Clinical Implications.Visc Med. 2020 Apr;36(2):129-136. doi: 10.1159/000506846. Epub 2020 Mar 13. Visc Med. 2020. PMID: 32355670 Free PMC article. Review.
-
KCa3.1 (IK) modulates pancreatic cancer cell migration, invasion and proliferation: anomalous effects on TRAM-34.Pflugers Arch. 2016 Nov;468(11-12):1865-1875. doi: 10.1007/s00424-016-1891-9. Epub 2016 Oct 17. Pflugers Arch. 2016. PMID: 27752766
References
-
- Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5: 685–694. - PubMed
-
- Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7: 68–77. - PubMed
-
- Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;114: 493–502. - PubMed
-
- Riegler M, Sedivy R, Sogukoglu T, Cosentini E, Bischof G, Teleky B, et al. Epidermal growth factor promotes rapid response to epithelial injury in rabbit duodenum in vitro. Gastroenterology. 1996;111: 28–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

